Gastrointestinal Stromal Tumours (GISTs) being most common non-epithelial tumours comprise a bunch of non-epithelial smooth muscle, mesenchymal alimentary tract tumours, appearing from the interstitial cells of Cajal of varying malignancy which specify KIT protein-CD117, a stem cell factor receptor. The mutations seen in GIST’s are most common in the proto-oncogene c-KIT; seen only in GIST. This gene is not seen in the other mesenchyamal tumours. Here, the mutated c-KIT helps in the growth and survival of this tumour by activation of the KIT receptor tyrosine kinase. The incident of GISTs is increasing in recognition and the increased rate of survival has made imaging seemingly important, not only for diagnosis, but also for monitoring the effects of treatment and detecting progression of the tumour. The clinical symtoms in this case series include pain abdomen, nausea, vomiting and constipation. Some cases can present as abdominal distension. The investigation of choice for these spectrum of diseases is currently Computed Tomography (CT), however many imaging techniques, such as Positron Emission Tomography (PET), fluorine 18 Fluorode-Oxyglucose (FDG), Magnetic Resonance (MR) imaging, and Ultrasonography (US) can also be used. This article describes the typical imaging findings of GISTs at initial presentation. Many cases present late and at a stage where surgery will not be of much use. However, Imatinib has showed a drastic response in prognosis and helps increasing the survival changes leading a normal life. This study helps us in identifying the pattern of the lesion and its enhancement patterns leading to early detection, thus aiding the surgeon or the oncologist to plan for treatment.
Introduction
The smooth muscle neoplasms of the GIT were termed stromal tumours by Clark and Mazur in 1983. Gastrointestinal Stromal Tumours (GIST) can make an appearance at any place within the GIT, as well as the mesentery, omentum, and retroperitoneum [1]. The abnormal proliferation of cells is due to the activation of mutated tyrosine kinase. Immunohistochemistry of these cells tests positive for CD117 and CD34. The production of KIT is due to the activation of the proto-oncogene [2]. Hereby, cases series are presented of GISTs.
Case 1
A 60-year-old female patient presented with complaints of pain abdomen, since one week and vomiting, from last two days. Here is a 60-year-old female patient with complaints of pain abdomen, since one week and vomiting, since two days. The CT [Table/Fig-1a] reveals a large ill-defined soft tissue density intraperitoneal mass lesion with hypodense necrotic areas within. On post-contrast study, the mass lesion is showing heterogeneous peripheral enhancement of solid component with non-enhancing necrotic areas. Anteromedially mass lesion is infiltrating greater curvature of stomach. Posteriorly, it is seen infiltrating body of pancreas with encasement of splenic artery; however head and tail of the pancreas are separately seen.
Shows CECT abdomen (a): Gross specimen (b): Cut open (c): Histopthology images (d) (H&E stain 100 x).
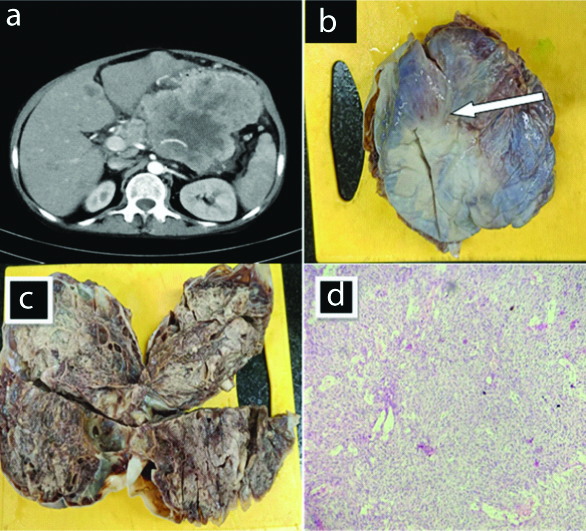
Gross anatomy [Table/Fig-1b] of the postoperative specimen reveals pale to darkish brown cystic tissue exhibiting congested external surface. Cut specimen [Table/Fig-1c] reveals solid and cystic areas. Solid areas displaying pale to gray white glistening areas. Cystic areas are stuffed with serosangionous fluid. Microscopy [Table/Fig-1d] shows tumour tissue organised in intersecting fascicles and bundles. Discrete cells have elongated nuclei having blunt ends. The patient was later referred to higher center for treatment.
Case 2
A 24-year-old male patient came with complaints of pain abdomen and distension since one month.
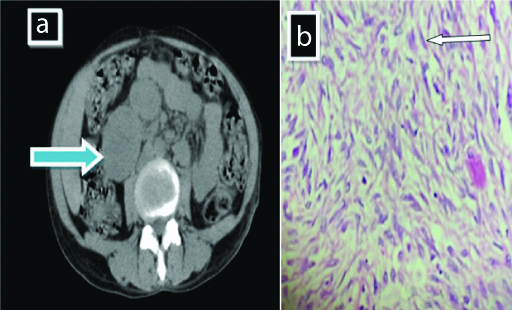
On CT [Table/Fig-2a], it confirmed a well defined distinct oval shaped moderate, heterogeneously enhancing mass lesions, interconnected with each other, seen inferior to the head of pancreas and in relation to second part of duodenum, abutting the uncinate process of pancreas and right proximal ureter, displacing it medially with loss of intervening fat plane. Microscopy [Table/Fig-2b] reveals spindle shaped cells having elongated nuclei with blunt ends. The nuclei are exhibiting pleomorphism having coarse nuclear chromatin and inconspicuous nucleoli and moderate quantity of pale eosinophilic cytoplasm. The patient was send to higher center for treatment.
Shows mass lesions (a) Interconnected with each other (blue solid arrow) with histopathology image (b) Showing spindle shaped cells having elongated nuclei with blunt ends (white arrow) (H&E stain 400 x).
Case 3
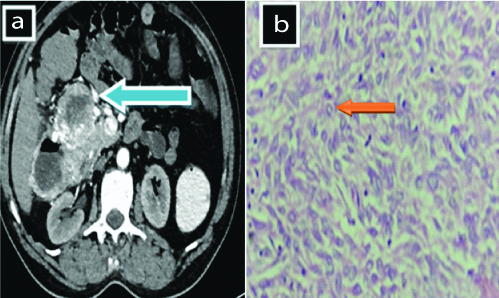
A 60-year-old male complains of abdominal pain and constipation, since two weeks. On CT [Table/Fig-3a], a large heterogenously enhancing multilobulated exophytic intraperitoneal mass lesion in the right subhepatic region of peritoneum appears to be arising from second and third part of duodenum from its anterior, and right lateral wall communicating with duodenal lumen at its right lateral wall with its extensions and adjoining infiltration to head of pancreas and hepatic flexure of ascending colon. Microscopy [Table/Fig-3b] reveals few round cells organised in sheets and nests having clear to eosinophilic cytoplasm suggestive of epithelioid cells. Patient was shifted to higher center for treatment.
CECT abdomen. a) showing an exophytic intraperitoneal mass lesion (solid blue arrow). b) shows few round cells organised in sheets and nests (H&E stain 400 x).
Case 4
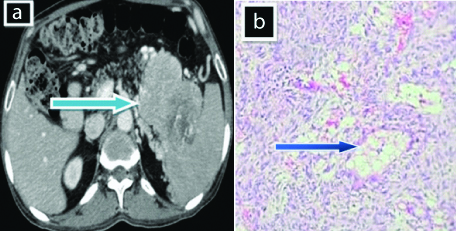
A 44-year-old male complaints of fullness of stomach and vomiting since one year, aggrevated since one week. On CT [Table/Fig-4a], there is evidence of large ill-defined heterogeneously enhancing exophytic mass arising from lesser curvature of stomach. The lesion is seen to abutting the tail of the pancreas and diaphragm displacing the tail of pancreas, superiorly. The mass has an endophytic component with few areas of necrosis within. Microscopy [Table/Fig-4b] shows myxoid change with perivascular hyalinisation. For treatment, patient was referred to higher center.
a) Shows ill-defined exophytic mass (solid blue arrow) arising from lesser curvature of stomach abutting the tail of the pancreas; b) Shows Myxoid change with perivascular hyalinisation (blue arrow) (H&E stain 100 x).
Case 5
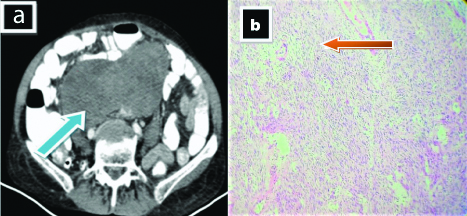
A 65-year-old female came with complaints of vomiting and abdominal distension since one month. On CT [Table/Fig-5a], there is evidence of an irregular lobulated exophytic soft tissue density mass lesion measuring about 81×88×70 mm noted in retroperitoneum in the midline anterior to aorta at L2-L3 vertebral body level abutting and displacing bowel loops and encasing superior mesenteric artery and its branches. On contrast administration, the mass lesion is showing heterogeneous enhancement with few non-enhancing necrotic areas within. Microscopy [Table/Fig-5b] shows cystic degeneration with cystic dilated spaces. The patient was later referred to higher center for treatment.
a) Shows irregular lobulated exophytic soft tissue density mass lesion (solid blue arrow); b) Shows cystic degeneration with cystic dilated spaces (green arrow) (H&E stain 400 x).
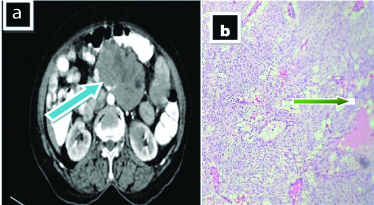
Case 6
A 65-year-old female presented with complaints of abdominal distention, constipation and vomiting, since one month. On CT [Table/Fig-6a], there is evidence of heterogenously enhancing large pelvic mass extending to the hypogastric, umbilical regions and right iliac fossa. The lesion encases and infiltrates sigmoid colon inflicting severe narrowing of the lumen; abuts and displaces the small pelvic bowel loops, rectum, urinary bladder, bilateral iliac vessels, bilateral psoas muscle tissues and bilateral lower ureters with loss of fats plane. There is mild fullness of bilateral renal pelvis noted. Microscopy [Table/Fig-6b] shows adjoining areas of congested blood vessels together with inflammatory cell infiltrates comprise of lymphocyte and mast cells. For treatment patient was referred to higher center.
a) Shows heterogenously enhancing large pelvic mass extending to the hypogastric, umbilical regions and right iliac fossa (solid blue arrow); b) Shows congested blood vessels together with inflammatory cell (red arrow) (H&E stain 400 x).
Discussion
Differential diagnosis: Many diseases resemble in the appearance of GIST. Few of the differential diagnosis are mentioned below.
Lymphoma stomach and small bowel is the most common site seen for lymphoma. It presents with circumferential wall thickening or aneurysmal dilatation of bowel loops, with abdominal lymphadenopathy. Lymphadenopathy is pathognomic feature of lymphoma differentiating from GIST [3].
Adenocarcinoma irregular or polypoidal growth, with or without intestinal obstruction is the general presentation of adenocarcinoma and is generally seen with lymphadenopathy, metastases, or ascites. On the other hand, GIST shows a predominant exophytic component without causing intestinal obstruction; rather it abuts the surrounding structures. CT helps in estimating the gastric as well as the extra-gastric extension of the diseases [4].
Peritoneal carcinomatosis: Multiple lesions with omental caking, ascites, and lymphadenopathy in the presence of a known primary like carcinoma of the ovary, stomach, etc., helps in identification of these lesions [5].
Carcinoid: These are well differentiated malignancies which are catergorised to a diverse group of tumour which seem to be originating from cells of endocrine system. These appear as a homogenously enhancing well defined bowel mass showing desmophytic reaction causing bowel obstruction
The presentation of GIST confines wide spectrum of symptoms like pain abdomen, vomiting and constipation. There is variable presentation on radiology which is often confused with abdominal lymphedenopathy. Enhancement of the lesion on CECT with few necrotic areas helps in differentiating with lesion.
In the recent times, the radiological diagnosis and pathophysiology of the GIST’s has given rise to vast interest after Imatinib, a C-KIT, PDGFRA and ABL inhibitor was discovered. Imatinib is a very effective, highly specific, chemotherapeutic agent [6,7]. The expression of tyrosine kinase (KIT) commonly involve small gut and stomach (rarely esophagus, mesentery and colon) [8]. Nevertheless, the mutations seen in GIST’s are of types, first mutations in KIT and Platelet-Derived Growth Factor Receptor Alpha (PDGFRA) seen in 80-85% cases and wild type GISTs seen in 15% cases, these type of mutations are not seen [9,10]. Some of these wild type GISTs are sporadic and others are associated with syndromes like neurofibromatosis, Carney-Stratakis syndrome and Carney triad [11].
Imatinib is a new chemotherapeutic agent used in the treatment of GIST. It is a molecularly targeted tyrosine kinase receptor blocker. Response to imatinib is usually good, with improved long-term survival [2,12]. The imaging features in patients showing response to imatinib include decrease in the density of the lesion, reduction in enhancement, and reduction in the number of nodules and number of vessels. The size of the lesion may increase or decrease. A better detailed evaluation of the disease might be accomplished by means of immunohistochemistry because the specificity is more and helps us in giving targeted treatment for the amelioration of the patient [13].
When a tumour extends beyond the serosal tumour seedling, a peritoneal implant occurs. Most of the peritoneal spread is by the tumour seedling during surgery/biopsy. Peritoneal deposits are often large discrete masses that look like the primary tumour and can extend up to the pelvis. The attenuation and enhancement pattern is similar to that of the primary lesion [14], similar finding are noted in case 1.
A study done by Patnaik S et al., shows multifocal lesions in the GIT findings which are similar to present study on Case 2 [15]. Many cases show variable patterns of metastatis. Hypervascular metastasis is unique feature.
Conclusion(s)
Gastrointestinal stromal tumours are rare soft tissue tumours which have unique presentations within the gasto-intestinal system. CT helps in better assessment in characterisation of the lesion based on its contrast enhancement. Any other distant metastases can also be assessed using CT. The histological presentation has specific characteristic features accounting for specificity in its prognosis. With the advancement in treatment of GIST in the form of Imatinib, the series presented a drastic improvement in the betterment and cure of the patient.
[1]. Panbude SN, Ankathi SK, Ramaswamy AT, Saklani AP, Gastrointestinal Stromal Tumour (GIST) from esophagus to anorectum–diagnosis, response evaluation and surveillance on computed tomography (CT) scanThe Indian Journal of Radiology & imaging 2019 29(2):13310.4103/ijri.IJRI_354_1831367084 [Google Scholar] [CrossRef] [PubMed]
[2]. King DM, The radiology of gastrointestinal stromal tumours (GIST)Cancer Imaging 2005 5(1):15010.1102/1470-7330.2005.010916361144 [Google Scholar] [CrossRef] [PubMed]
[3]. Lewis RB, Mehrotra AK, Rodríguez P, Manning MA, Levine MS, From the radiologic pathology archives: Gastrointestinal lymphoma: Radiologic and pathologic findingsRadiographics 2014 34(7):1934-53.10.1148/rg.34714014825384294 [Google Scholar] [CrossRef] [PubMed]
[4]. Ba-Ssalamah A, Prokop M, Uffmann M, Pokieser P, Teleky B, Lechner G, Dedicated multidetector CT of the stomach: spectrum of diseasesRadiographics 2003 23(3):625-44.10.1148/rg.23302512712740465 [Google Scholar] [CrossRef] [PubMed]
[5]. Diop AD, Fontarensky M, Montoriol PF, Da Ines D, CT imaging of peritoneal carcinomatosis and its mimicsDiagnostic and Interventional Imaging 2014 95(9):861-72.10.1016/j.diii.2014.02.00924631039 [Google Scholar] [CrossRef] [PubMed]
[6]. Sripathi S, Rajagopal KV, Srivastava RK, Ayachit A, CT features, mimics and atypical presentations of gastrointestinal stromal tumour (GIST)The Indian Journal of Radiology & Imaging 2011 21(3):17610.4103/0971-3026.8536422013291 [Google Scholar] [CrossRef] [PubMed]
[7]. Foo WC, Liegl-Atzwanger B, Lazar AJ, Pathology of gastrointestinal stromal tumoursClinical Medicine Insights: Pathology 2012 5:CPath-S9689.10.4137/CPath.S968922855636 [Google Scholar] [CrossRef] [PubMed]
[8]. Marrari A, Wagner AJ, Hornick JL, Predictors of response to targeted therapies for gastrointestinal stromal tumoursArch Pathol Lab Med 2012 136:483-89.10.5858/arpa.2011-0082-RA22229850 [Google Scholar] [CrossRef] [PubMed]
[9]. Poveda A, García Del Muro X, Antonio López-Guerrero J, Cubedo R, Martínez V, Romero I, Tumour review GEIS guidelines for gastrointestinal sarcomas (GIST)Cancer Treat Rev 2017 55:107-19.10.1016/j.ctrv.2016.11.01128351781 [Google Scholar] [CrossRef] [PubMed]
[10]. Tirumani SH, Jagannathan JP, Krajewski KM, Shinagare AB, Jacene H, Ramaiya NH, Imatinib and beyond in gastrointestinal stromal tumours: A radiologist’s perspectiveAm J Roentgenol 2013 201:801-10.10.2214/AJR.12.1000324059369 [Google Scholar] [CrossRef] [PubMed]
[11]. Tirumani SH, Baheti AD, Tirumani H, O’neill A, Jagannathan JP, Update on gastrointestinal stromal tumours for radiologistsKorean Journal of Radiology 2017 18(1):84-93.10.3348/kjr.2017.18.1.8428096720 [Google Scholar] [CrossRef] [PubMed]
[12]. Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C, Gastrointestinal stromal tumour: Role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinibRadiographics 2006 26:481-95.10.1148/rg.26205509716549611 [Google Scholar] [CrossRef] [PubMed]
[13]. Heinrich MC, Rankin C, Blanke CD, Demetri GD, Borden EC, Ryan CW, Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumours with next-generation sequencing resultsJAMA Oncol 2017 3:94410.1001/jamaoncol.2016.672828196207 [Google Scholar] [CrossRef] [PubMed]
[14]. Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Gastrointestinal stromal tumours: The incidence, prevalence, clinical course and prognostication in pre-imatinib mesylate era: A population based study in western SwedenCancer 2005 103:821-29.10.1002/cncr.2086215648083 [Google Scholar] [CrossRef] [PubMed]
[15]. Patnaik S, Jyotsnarani Y, Rammurti S, Radiological features of metastatic gastrointestinal stromal tumoursJournal of Clinical Imaging Science 2012 2:4310.4103/2156-7514.9917722919557 [Google Scholar] [CrossRef] [PubMed]