Hyperlipidemia is an essential feature of Nephrotic syndrome (NS) in children. There is an alteration in the lipoprotein metabolism in NS with marked elevation of all apolipoprotein B (apoB) containing lipoproteins such as Very-Low-Density Lipoproteins (VLDL), Intermediate-Density Lipoproteins (IDL), Low-Density Lipoproteins (LDL), and lipoprotein (a) {Lp(a)}. High-Density Lipoproteins (HDL) are reported to be unchanged or reduced [1].
Lp(a) is an LDL-like lipoprotein that consists of an LDL particle to which the glycoprotein apolipoprotein(a) {apo(a)} is attached. Children with NS have several folds increase in serum Lp(a) concentrations compared to controls [2]. In fact, NS is the condition in which the highest levels of Lp(a) have been reported [3].
Standard therapy with corticosteroids induces remission in steroid sensitive NS, but the majority of patients Suffer Infrequent Relapse (IFR) or Frequent Relapse (FR) some become steroid dependent (SD) [4]. Prolonged steroid therapy has several negative side-effects. Thus, prediction of risk of future relapses and steroid dependency provides the paediatric nephrologist opportunity to individualise treatment strategies and enable counseling [5-7], however, this remains a challenge even today [8]. Several clinical and laboratory parameters have been studied as predictors such, as age of onset, microscopic haematuria, time taken for remission from start of steroid therapy but with inconsistent results [9-11].
Lp(a) levels increases dramatically in relapse and reduces in remission but still remaining higher than healthy controls at all stages. Measurement of Lp(a) concentration has been suggested to monitor the disease course in children; especially to detect complications [12]. One particular retrospective study has shown that high levels of Lp(a) can predict future relapses [13]. The present study evaluated the potential of Lp(a), measured on admission, for the prediction of relapse/steroid dependency, in the first year after initial diagnosis.
Materials and Methods
The present study was undertaken as a prospective observational case control study at Institute of Child Health, a tertiary care paediatric teaching hospital of Kolkata between Dec 2011 and Dec 2012.
Inclusion criteria: All children below 18 years of age with first episode of primary idiopathic NS admitted in the paediatric ward or visiting the outpatient nephrology clinic were recruited following written informed consent from parents.
Exclusion criteria: Patients who had previously undergone management of NS, or diagnosed with secondary NS, anaemia, raised liver enzymes above normal range or with known liver disease and/or active infections (such as pneumonia, infective hepatitis, urinary tract infection etc.,).
Patients, who did not respond to standard doses of steroids within 4 weeks, were categorised as steroid resistant and also excluded. Recruited patients remained under the care of the treating paediatric nephrologists of the institute for standard management and monitoring. Patients who responded to predisolone and reported urine albumin nil or trace by dipstick (or proteinuria <4mg/m2/h) for three consecutive early morning urine specimens were defined to be in remission. The days taken to achieve the first remission was recorded. A minimum follow-up of 12 months was essential to record overall progress. A relapse was defined as urine albumin 3+ or 4+ on dipstick (or proteinuria >40 mg/m2/h) for three consecutive early morning specimens, having been in remission previously. Depending on relapse nature and frequency in the first year, patients were segregated as: no relapse (NR), IFR, FR and SD. Patients who did not relapse during follow-up were classified as NR. IFR was defined as three or less relapses in 12 month. FR was defined as the occurrence of >3 relapses in 12 months, and steroid dependence as the presence of two consecutive relapses while on tapering doses of prednisolone [14]. NR and IFR were defined as mild disease and FR and SD as severe disease. Patients who did not comply were considered dropouts and excluded from the study. Fifteen healthy volunteers visiting the hospital were requested to donate blood for lipid and Lp(a) levels which were tested and documented. The study protocol was approved by the Institutional Ethics Committee (No: ICH/525/MM/2011).
Sample size calculation: To obtain a clinically significant difference in levels of Lp(a) between groups, calculations from the prior retrospective study [13] suggested that with a two-sided significance of 0.05 and a power of 0.8, a total of 10 patients in each group would be required (the groups in the prior study were IFR vs FR). Considering that we did not know at start which relapse group the patients would fall into, as well as potential dropouts, we initially recruited a total of 56 NS children. At the end of study, we had 22 children in the NR+IFR group and 14 children in the FR+SD group. Informed consent was obtained from parents or legally authorised representative (LAR) of children enrolled in the study. Where applicable assent was also obtained.
Laboratory tests: In addition to standard biochemical tests performed for diagnosing NS, blood samples (clotted blood) were drawn at presentation, and processed for serum Lp(a) (mg/dL), total cholesterol (mg/dL), High Density Lipoprotein (HDL) (mg/dL), Low Density Lipoprotein (LDL) (mg/dL), and triglycerides (mg/dL), respectively, from both patient and control arms, as per standard procedures, and if not tested the same day, preserved at -20°C.
All parameters were estimated on automated platforms in the clinical chemistry laboratory of the Institute of Child Health, Kolkata. These investigations were already in routine use before the study began and are evaluated for consistent performance with robust internal and external quality assurance programme. Serum Lipoprotein A, mg/dL (immunoturbidimetry), total cholesterol (enzymatic colorimetry), HDL, mg/dL (homogenous enzymatic colorimetry), triglycerides (enzymatic colorimetry), serum albumin (bromocresol green), urine protein (turbidimetry) and creatinine (enzymatic colorimetry) were measured on the Roche Integra 400 Plus chemistry analyzer. LDL was calculated using the Friedewald equation [15]. The assays were validated for reproducibility using Quality Control (QC) materials from Roche Inc. Satisfactory coefficient of variation (CV%) were obtained for all parameters calculated from QC data run at significant levels of measurement.
Data collection: The following data were collected prospectively, using a standardised case record form- age at onset of disease, gender, medical history, response to treatment, time to resolution of proteinuria and baseline laboratory data such as haemoglobin, serum creatinine, albumin, aspartate aminotransferase, (AST) alanine aminotransferase (ALT), C-reactive protein (CRP) levels and urinary protein creatinine ratio. Follow-up data collected during clinic visits and/or through telephonic interviews, included- number of relapses within 12 months, steroid dependence, and use of steroid sparing agents.
Statistical Analysis
General characteristics of the population were described. Data for continuous variables are presented as medians and range and categorical variables as percentages. Normally distributed data are expressed as mean values±SD and non-normal data as medians and quartiles. Statistical analysis was performed using statistical software Meldcalc 8.0, Mariakerke, Belgium. Differences of laboratory findings between groups were assessed by non-parametric Mann-Whitney U-test. Evaluation of correlation was determined with Spearman rho test. The Kruskal Wallis non-parametric test with the Dunn’s post-test was performed to find out significant difference in the mean Lp(a), haemoglobin, creatinine, protein and albumin of the NR, IFR, and SD groups. A two-tailed p-value less than 0.05 were considered to be statistically significant.
Results
Of 36 study subjects, (median age 3 years, 19 males), there were 15NR, 7IFR, 2FR and 12SD, thus there were 22 children with mild disease and 14 children with severe disease. Fifteen healthy children were tested as controls.
Clinical features: Comparison of baseline features between the mild and severe disease groups of NS children revealed that, at onset, IFR or SD group were significantly younger in age than the non-relapsers. Other characteristics such as male-female ratio, and days to remission after first episode did not differ between the groups presented in [Table/Fig-1].
Demographic and laboratory data compared between the NS groups.
| Studied parametres | Group 1 Non relapsers (NR) n=15 | Group 2 Infrequent relapsers n=7 | Group 3 Steroid dependence n=12 | p-value |
|---|
| Median age at onset, years (IQR) | 4.3 (2.8-6.5) | 2.5 (2.0-3.0) | 2.8 (2.0-3.5) | p<0.05 (with Group 2 and 3) |
| Male | 5 (33%) | 5 (71%) | 8 (67%) | |
| Hematuria at onset | None | 1/7 | 1/12 | |
| Urine protein creatinine ratio, g:g | 8.8 (3.5-12.7) | 12.4 (10.6-14.6) | 11.9 (6.1-13.0) | 0.25 |
| Median days to remission, first episode NS (IQR) | 12 (10-18) | 12 (9-14) | 16 (9-26) | 0.65 |
| Heamoglobin, g/dL | 12.9 (11.5-13.2) | 13.3 (11.8-14.6) | 13.2 (12.8-13.9) | 0.08 |
| Creatinine, mg/dL | 0.27 (0.20-0.39) | 0.20(0.20-0.22) | 0.20 (0.20-0.26) | 0.29 |
| Total protein, g/dL | 4.0 (6.7-4.3) | 3.8 (3.3-3.9) | 3.6 (3.3-4.2) | 0.41 |
| Albumin, g/dL | 1.3 (1.5-2.0) | 1.3 (1.1-1.5) | 1.5 (1.4-1.8) | 0.07 |
Frequent relapses (n=2), not included in analysis; # Mann Whitney U test used for differences in age; p<0.05 considered significant; Kruskal Wallis test with Dunn post test for differences in tests; NS: Nephrotic syndrome; IQR: Interquartile range
Laboratory features at presentation: Urinalysis for haematuria, and proteinuria was comparable between groups. In addition, laboratory data such as, concentration of haemoglobin, serum creatinine, total protein and albumin did not differ between the two groups.
Lipid profile analyses: The mean Lp(a) of the entire NS group (165.2±120.4 mg/dL) was higher compared to controls (30.52±21.9 mg/dL) (p<0.0001). All the lipid parameters except HDL-cholesterol were significantly higher in the NS group. Total cholesterol (432.2±129.6 mg/dL) LDL Cholesterol (326.4±120.6 mg/dL) and triglyceride levels (304.4±157.8 mg/dL) of the NS group were significantly higher when compared to controls (p<0.0001) given in [Table/Fig-2].
Comparison of lipids amongst the three nephrotic syndrome (NS) groups and controls.
| Parametres | Control healthy volunteers n=15 | Group 1 Non relapsers n=15 | Group 2 Infrequent relapsers n=7 | Group 3 Steroid dependence n=12 | p-value Comparision of Group 3 with Group 1 and 2 |
|---|
| Cholesterol, mg/dL | 131.3±39.01 | 375.7±112.4 | 480.7±113.3 | 474.7±139.6 | p=0.05 (with Group 1)p=1.0 (with Group 2) |
| HDL-Cholesterol, mg/dL | 50.27±17.65 | 49.4±38.76 | 34.14±27.52 | 45.67±22.64 | p=0.84 (with Group 1)p=0.37 (with Group 2) |
| LDL-Cholesterol, mg/dL | 88.85±32.97 | 269.0±94.81 | 388.6±123.6 | 365.9±125.2 | p=0.02 (with Group 1)p=0.04 (with Group 2) |
| Triglyceride, mg/dL | 110.5±46.48 | 236.3±160.7 | 319.9±141.3 | 317.9±173.5 | p=0.71 (with Group 1)p=0.83 (with Group 2) |
| Lp(a), mg/dL | 30.52±21.9 | 129.7±120.1 | 143.8±106.8 | 222.0±115.7 | p=0.02 (with Group 1)p=0.16 (with Group 2) |
HDL: High density lipoprotein; LDL: Low density lipoprotein; Lp(a): Lipoprotein a; Mann whitney U test used for differences; p<0.05 considered significant
Lp(a) showed significant correlation (Spearman-rho) with albumin (p=0.0062, r=0.47), but showed no correlation with protein (p=0.0725, r=0.35), Total cholesterol, HDL, LDL, or triglycerides. Plasma Lp(a) concentrations were not associated with proteinuria (p=0.27, r=-0.21). It was unrelated to the number of days to remission after initial steroid therapy and also with the frequency of relapses.
Within the NS group, comparison of Lp(a) in the sub-groups revealed that the SD patients (Group 3) had a significantly high Lp(a) at onset of disease when compared to non relapsers (p=0.0264) (Group 1) [Table/Fig-3]. There was however no significant difference when compared with IFR (p=0.72) (Group 2). Comparison of all the groups {FR group was excluded, (n=2) due to low numbers for statistical analyses} by Kruskal Wallis test showed no significant difference between the groups (p=0.07). Comparision of Lp(a) between nonrelapsers and relapsing group (NR vs IFR+FR+SD) showed no significant difference (p=0.14).
Comparision of Lipoprotein (a) concentrations (mg/dL) in controls (normal group), non relapsers, infrequent relapsers and steroid dependent NS children at the onset of disease. NS: Nephrotic syndrome
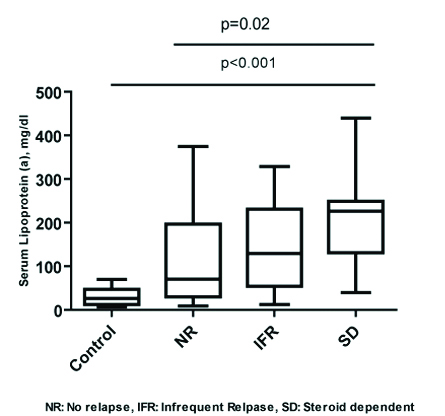
The total cholesterol and LDL cholesterol levels in the SD patients were also significantly higher at onset when compared to the non relapsers (p=0.05, p=0.04, respectively); in the IFR, the total cholesterol was higher but significance was missed by a slight margin (p=0.06), while the LDL cholesterol was significantly higher (p=0.02) as also observed with the non-relapsers group. HDL Cholesterol and triglycerides in the SD group were not significantly elevated when compared with nonrelapsers (p=0.71) or with IFR (p=0.62) presented in [Table/Fig-2].
Discussion
The present study reports the high concentrations of Lp(a) in idiopathic steroid sensitive NS in children when compared to healthy controls. Furthermore, the Lp(a) levels are greatly increased at onset of the disease in NS children who later show steroid dependence. Thus, Lp(a) could serve as a predictive biomarker for steroid dependence in idiopathic steroid sensitive NS, and be used as a guide towards early use of steroid sparing agents. Several studies have shown high levels of lipoproteins in NS, but that Lp(a) could predict steroid dependence has not been proposed earlier. Kawasaki Y et al., in their retrospective analysis have shown that high levels of Lp(a) can predict future relapses in these children and thus should be well documented [13]. The predictive role of high Lp(a) levels at onset of NS could be exploited to manage and treat Steroid Sensitive Nephrotic Syndrome (SSNS) with steroid sparing agents in children who present to the clinic at a comparatively younger age.
Various authors have studied multiple factors that predict relapses in SSNS children. Sinha A et al., have summarised that FR include, age younger than 3 year at onset, delayed time to remission after seven-nine days and occurrence of an early relapse (in the first six months after initial treatment) as important factors for predicting relapses in Indian NS children [7]. Yap HK et al., identified that delayed time to remission of more than nine days and concomitant Upper Respiratory Tract Infection (URTI) during relapses were significant predictors of steroid dependency [16]. The present study has shown that children who suffered relapses or became SD to maintain remission were younger in age. The time to initial remission was also more than nine days in these children. Authors could not account for infections as it was not specifically captured.
Lp(a) is a circulating plasma lipoprotein that consists of cholesterol, phospholipids and apolipoprotein B-100 (i.e., a LDL particle) to which is attached a highly polymorphic glycoprotein, apolipoprotein a. Lp(a) levels are higher in Indians, in comparision to their caucasian counterparts [17], and is an independent risk factor for atherosclerosis and thrombosis [18]. About 80% of amino acids in apo(a) are homologous with those of plasminogen suggesting a role in thrombotic occlusions. Furthermore, Lp(a) disorder also contributes to the development and progression of renal complications in NS.
The Lp(a) level in the healthy control group (mean: 30.52±21.9 mg/dL) is albeit higher when compared with published data in Caucasian children. There is yet no published data of Lp(a) levels in healthy Indian children; it is important to establish biological reference intervals as Lp(a) levels are higher in South East Asian populations and levels >30 are considered an important contributor of atherosclerosis [19].
In this study, serum concentrations of serum lipids including total cholesterol, triglyceride, LDL, VLDL-cholesterol, and Lp(a) in patients with NS were higher than those in the control group. This is tune with the findings of quantitative increase of all apoB containing lipoproteins in NS [1,12,13,20,21]. However, HDL-cholesterol was unchanged in all the groups. While most authors have reported similar observations [2,13,22], other groups did find a reduction in HDL levels as reviewed by Vaziri ND [23]. The possible explanations of increase in apoB lipoproteins in NS have been ascribed to an increase rate of synthesis in the liver in response to decrease in plasma albumin levels due to proteinuria [22]. While some studies have indeed reported a negative correlation of Lp(a) with proteins [2,13], the present study shows that Lp(a) was positively correlated. Another study found no correlation of Lp(a) with albumin [21]. This can be explained by the in-depth review of Wheeler DC and Bernard DB, who have clearly written that there is no clear link between the increased hepatic synthesis of lipoproteins and albumin, and the reasons may be multifactorial as established by in vivo and animal experiments [24]. This study also finds no correlation with serum Lp(a) level and proteinuria in the NS subjects as has been reported elsewhere [2,13].
The pathophysiology of higher Lp(a) levels in NS children compared to the age and sex match control group can be somewhat reasoned by the experiments of Kronenberg F et al., who have elegantly shown that Lp(a) levels in plasma vary widely due to size polymorphism of apo(a) moiety [25]. Low Molecular Weight (LMW) isoforms of apo(a) are responsible for high serum Lp(a) concentrations and vice versa for high molecular weight isoforms. Children with SSNS with LMW isoforms who have higher Lp(a) concentrations tend more often toward a nephrotic course of kidney disease. Since apo(a) isoforms were not determined nor were the histopathology of the NS captured in this study, it would be difficult to assign the reasons for high Lp(a) in FR and SD NS compared to nonrelapsers.
The interest in Lp(a) has been resurrected with studies trying to fathom the genetic basis of its pathophysiology in coronary heart disease and its possible implications in NS [26]. A recent publication [8] states that in children, demographic, clinical, and family reported characteristics, specifically steroid sensitivity, are not useful in predicting relapse rates or long-term remission in idiopathic NS. There is a renewed interest in biomarkers for prediction such as atypical IgM on T cells [27] to predict relapse and steroid dependence, or urinary epidermal growth factor [28] to predict kidney function in NS. High value of Lp(a) identified in the present study study may be predictive in this regard. Our findings also suggest that Lp(a) may play an important role in the pathogenesis of FRNS.
A high Lp(a) level would alert physicians caring for these patients to monitor them closely, use steroid sparing agents early, and allow them to appropriately counsel patients and the caretakers. Furthermore, the pattern of dyslipidemia in NS in Indian children needs to be studied more specifically for its affect on atherosclerosis given that both serum Lp(a) levels and incidence rate of NS is much higher in the population of present study.
Limitation(s)
This study was limited by a small sample size and underpowered due to dropouts, especially of non-inclusion of FR in the data set, and inability to assay apo(a) isoforms in the NS subjects.
Conclusion(s)
Serum Lp(a) concentrations are higher in SD idiopathic SSNS children at onset of disease compared to those who suffer no relapses. This suggests for a predictive role of serum Lp(a) in determining early treatment strategy to avoid complications of relapsing INS and hence should be included in the early diagnostic workup of INS. However, the pilot study attempts to report a possible role of Lp(a) levels in predicting steroid dependence NS children at onset, future research is advisable in a larger sample size and of greater duration. Further research could be done to discern the isoforms of Lp(a) levels in NS with worse prognosis.
Declaration: The abstract of the study is published as Conference Abstract in the Clinical Biochemistry Journal as part of Abstracts of the XIIIth International Congress of Pediatric Laboratory Medicine in June 2014, Volume 47, Issue 9.
Frequent relapses (n=2), not included in analysis; # Mann Whitney U test used for differences in age; p<0.05 considered significant; Kruskal Wallis test with Dunn post test for differences in tests; NS: Nephrotic syndrome; IQR: Interquartile range
HDL: High density lipoprotein; LDL: Low density lipoprotein; Lp(a): Lipoprotein a; Mann whitney U test used for differences; p<0.05 considered significant