Introduction
Procedural analgesia use in neonatal circumcision is not widespread in the developing world. An easy-to-administer, adequate and safe analgesia will encourage usage in neonatal circumcision. Orally administered ketamine may prove effective and safe, and may encourage procedural analgesia use in neonatal circumcision.
Aim
To determine the analgesic efficacy of oral ketamine in Plastibell® neonatal circumcision.
Materials and Methods
A hospital based randomised double blind controlled study was conducted at the paediatric surgery unit of the hospital, from March 2015 to December 2015. Total 121 neonates were sequentially recruited, and randomised into two groups. Group A received oral ketamine, and Group B received plain syrup (placebo) as procedural analgesia. Continuous pulse oximeter monitoring was done before, during and immediately after the procedure. The pre-procedural and intra-procedural peripheral oxygen saturation (SpO2) and Pulse Rate (PR) were determined at the various stages. Also, the Neonatal Infant Pain Scale (NIPS) scores were assessed during the stages of the procedure. Differences in mean scores were analysed. Mann-Whitney U test and Independent t-test were used to compare means of continuous variable, while Fisher’s exact test was used to compare categorical variables. Significance was set at p<0.05.
Results
Sixty-one neonates received oral ketamine, while 60 received placebo. The intraoperative mean SpO2 were lower in the placebo group and significant at the tying stage with p=0.022. The mean intraoperative PR was higher in the placebo group and significant at dorsal-slit, tying and excision stages (p<0.05). The mean intraoperative NIPS scores were significantly higher in the placebo group.
Conclusion
Oral ketamine provides effective and safe analgesia for neonatal Plastibell® circumcision in comparison to placebo.
Introduction
There has been a plethora of anaesthesia/analgesia used for neonatal circumcision. These include Eutectic Mixture of Local Anaesthetics (EMLA), Dorsal Penile Nerve Block (DPNB), penile ring block, caudal block, general anaesthesia and paracetamol [1,2]. There are also non-pharmacological agents such as pacifiers, and sucrose/glucose on a pacifier, rocking, massage, facilitated tucking, cuddling, music and other sounds (intrauterine heart-beat) [3]. These agents have not been shown to be very effective. The use of EMLA® for neonatal circumcision has not been entirely satisfactory [4]. DPNB provides fair analgesia but is limited for not taking care of the nerves on the ventral surface of the penis [1]. The ring block seems to be the best of the local analgesics, however it still does not eliminate all pain probably because of the complex pain pathway of the penis and prepuce [5,6]. Caudal block and general anaesthesia will obviously ablate the pain in neonatal circumcision, but seems rather a cumbersome method for just a “minor procedure”. Paracetamol is best reported as a postprocedural analgesic [2].
Some analgesia like parenteral opioid, parenteral ketamine and thiopental as a component of general anaesthesia, have not been widely reported in neonatal circumcision. Therefore, there is room for trials of more analgesic agents such as ketamine in neonatal circumcision [7].
Despite the plethora of available analgesics, the usage has been low, especially in low and middle income countries, notwithstanding the many deleterious effects of unmitigated procedural pain. Such deleterious effects include stronger response to future painful stimulus; increased stress evidenced by raised cortisol level, reduced oxygen saturation and increased heart rate; alteration to sleep-wake cycle; and reduced response to parents [8-12]. The reason adduced has been reluctance of practitioners to use analgesics in neonates from safety concerns [13]. Some practitioners are reluctant in inflicting needle stick pain. Many consider the procedure fast and quick and not worthy of effecting pain in order to provide the analgesia [14]. However, the pain of circumcision outlasts the procedure itself, and therefore requires intra-procedural analgesia. It was in the quest to avoid painful injections for the provision of procedural analgesia, that the concept of orally administered ketamine was developed [8,11,12].
Ketamine has relatively short distribution and elimination half-lives: the alpha-elimination phase lasts only a few minutes, and the beta-elimination half-life is 2-3 hours. It is metabolised in liver [15,16]. Its primary metabolite norketamine is only one-third to one-fifth as potent as the original compound but may be involved in the prolonged analgesic actions of ketamine. Bioavailability by oral or rectal routes is only 16%. Following oral administration of ketamine, norketamine levels are three times higher than with intravenous administration. The compound interacts with multiple binding sites, including N-Methyl-D-Aspartate (NMDA) and non-NMDA glutamate receptors. Ketamine produces bronchodilatation, ionotropic and chronotropic effects, dissociative anaesthesia, pro- and anti-convulsant effects, analgesia, stimulates gastrointestinal tract, and increases muscle tone [16]. Ketamine, in all age groups is used for induction and maintenance of anaesthesia, sedation, analgesia, in asthmatic patients and recently for pre-emptive analgesia and neuroprotection [7,15,17].
To the best of our knowledge, there has not been a reported use of oral ketamine as analgesia for neonatal circumcision. However, there has been use of oral ketamine to provide analgesia alone and/or sedation in the children undergoing other surgical procedures [17-20]. Also, there is no reported study on the use of oral ketamine in neonates, however the parenteral use of ketamine in newborns to relieve procedural pain is common in the literature [7,21].
Thus, the aim of this study was to determine the analgesic efficacy and safety of oral ketamine in neonatal circumcision, compared to a placebo. Primary outcome measures were a significant lower mean PR and higher mean SpO2 in neonates who received oral ketamine compared to those who received plain syrup. The secondary outcome measures were lower NIPS score and insignificant side-effects in the oral ketamine group compared to the placebo group.
Materials and Methods
This was a hospital based randomised double blind controlled study, conducted at the paediatric surgery unit of the hospital, from March 2015 to December 2015. It involved sequential recruitment of neonates that presented for circumcision at the clinic, and whose parents/guardians gave consent. Ethical approval was obtained from the hospital health research ethical board (NAUTH/CS/66/Vol.5/82).
An average of 230 circumcision procedures is done in infants per year in the centre. Fisher’s formula was used to determine the sample size of 121. Using the formula for a population less than 10,000:
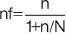
Where, nf=the desired sample size (when population is less than 10,000)
n=sample size for population greater than 10,000=(Z2pq)/d2
N=estimate of population size=number of neonates who had circumcision at the centre yearly=233
z=the standard normal deviate, set at 1.96, which corresponds to the 95% confidence level.
p=proportion of male neonates in Nigeria (target population). Here, 84% was used as this is the prevalence for male neonates who underwent circumcision from previous studies [22-24]. Therefore the available prevalence=0.84
q=1-p (1-0.84)=0.16
d=degree of accuracy desired, 0.05
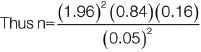
n=206.52
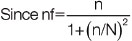
=(206.52)/{1+(206.52/233)}
=109.85
Making provision for attrition rate of 10% which is 10.99
=109.85+10.99
=120.84
=121
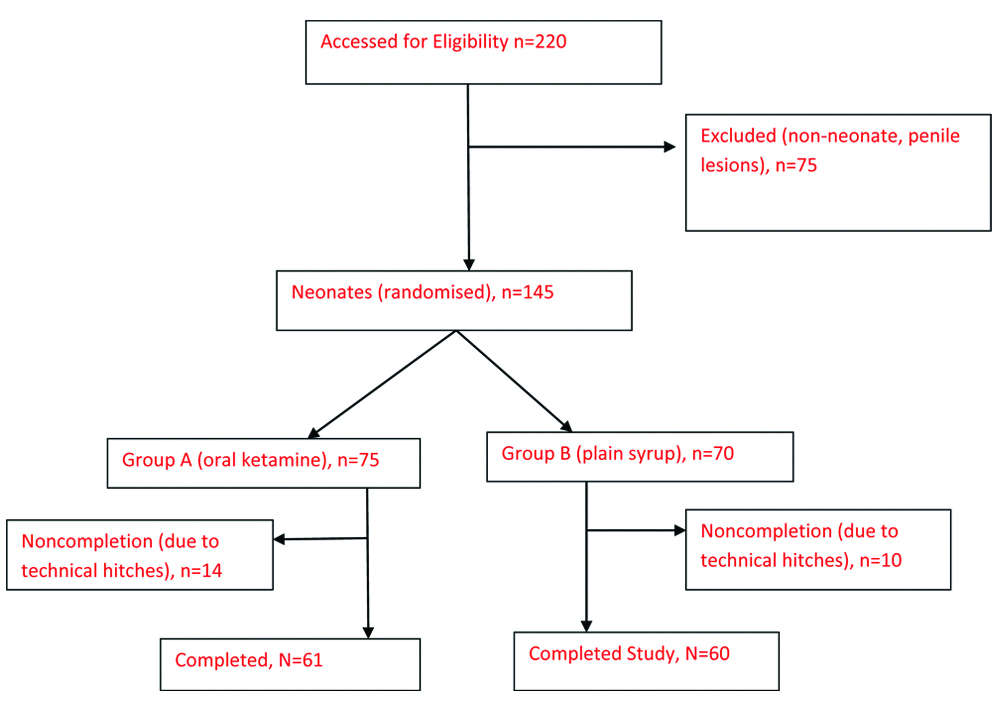
All term male neonates were included in the study. Excluded from the study were neonates delivered pre-term, with penile lesions like rashes/ulcers and congenital penile anomalies like hypospadias, or epispadias. Every week, recruited neonates [Table/Fig-1] were randomised into two groups, A and B by a simple ballot method, picking from a bag containing equal amount of tags A and B. Each was assigned to the group of its tag. One assistant was responsible for the balloting and another for administering the medications. These two personnel did not participate in the actual procedure and data recording. The group and medications were filled into the proforma at the end of the procedure.
The neonates in group A received 10 mg/kg body weight of oral ketamine (10 mg/mL) [16]. The neonates in group B received a placebo, simple sucrose (66.7% w/w) at 1 mL/kg of body weight. This ensured that neonates of similar weight received same volume of “syrup”. The oral ketamine was prepared by the compounding section of the hospital pharmacy using simple sucrose, as a base and sweetener, without a preservative. The operating room was made conducive for the neonates by having the air-conditioner switched off and the cleansing solutions warmed to reduce confounding effect on parameters of outcome measures. There was no restriction on feeding.
Two senior registrars from the anaesthesia department of the hospital, who were conversant with the study, provided Monitored Anaesthesia Care (MAC). Part of their duty was to observe for the known side-effects of ketamine that may be life-threatening such as apnea, laryngeal spasm, and aspiration. They had facilities and instruments to manage such side-effects whenever the need arise. Such facilities included but not limited to: anaesthetic machine, piped oxygen, laryngoscope, various sizes of endotracheal tubes, patient monitor, suction machine, atropine, adrenaline, suxamethonium, pancuronium etc. The circumcision procedure was carried out 30 minutes after oral medication administration. The surgeon, parent or caregiver, the anaesthetists and the assistant that recorded the details were all blinded to the type of oral medication received by the neonates.
The neonates were placed on continuous pulse oximetry (MMED, MD300A, China; 12110/000189; 2012) monitoring before, during and after the procedure. The baseline SpO2 and PR were recorded before the onset of the procedure. The SpO2, PR and NIPS score were recorded at the end of adhesiolysis, dorsal slit, tying and excision. The neonates were also observed for complications such as laryngeal spasm, apnoea, aspiration, increased salivation, and vomiting. These were recorded on the proforma.
Statistical Analysis
Data analysis was done using SPSS version 21. The statistical differences in the mean age, weight, pre-procedural SpO2 and PR of both groups were compared using t-test for confounding factors. The significance of the difference in the mean SpO2 and mean PR at the stages of the procedure for the different syrups were analysed using Mann-Whitney U test. Independent t-test was used to assess the significance of the difference in mean NIPS score of both groups. Fisher’s exact test was used to compare categorical variables.
Results
A total of 121 subjects were recruited in this study, after 220 were assessed: 61 in group A and 60 in group B [Table/Fig-1]. The age and weight distributions of the neonates are shown in [Table/Fig-2].
Comparison of mean ages, weights, and preoperative SpO2 and Pulse Rate (PR).
| Characteristics | Group A Mean (SD) | Group B Mean (SD) | p-value (t-test) |
|---|
| Age of neonates (days) | 15.2 (5.7) | 15.0 (5.7) | 0.509 |
| Weight of neonates (kg) | 3.67 (0.45) | 3.63 (0.54) | 0.692 |
| Preoperative SpO2 (%) | 96.8 (1.8) | 97.3 (1.5) | 0.089 |
| Preoperative PR | 140.2 (14.7) | 141.0 (10.3) | 0.729 |
p<0.05 is statistically significant
The mean intra-procedural SpO2 for the group A and group B were 95.5% and 95.6 (adhesiolysis); 95.3% and 94.4% (dorsal slit); 94.6% and 93.5% (tying); and 95.9% and 94.8% (excision), respectively. There were no significant differences in the mean SpO2 levels between the two groups during the adhesiolysis, dorsal slit and excision stages of the procedure. However, the mean SpO2 levels were significantly lower in the placebo group during the tying stage (p=0.022). The mean PR values were significantly higher in the placebo group during the dorsal slit, tying and excision stages of the operation (p-values were <0.001, <0.001 and <0.001, respectively). There was, however, no difference in the PR of the two groups during the adhesiolysis stage (p=0.077) [Table/Fig-3].
Comparison of mean intraprocedural SpO2 (%) and Pulse Rate (PR) (b/min)
| Stage of procedure | | Group A Mean (SD) | Group B Mean (SD) | p-value (Mann Whitney-U test) |
|---|
| Adhesiolysis | (SpO2) | 95.5 (2.3) | 95.6 (3.1) | 0.457 |
| (PR) | 158.1 (26.8) | 162.8 (11.3) | 0.077 |
| Dorsal Slit | (SpO2) | 95.3 (2.2) | 94.4 (3.1) | 0.060 |
| (PR) | 165.5 (16.6) | 175.0 (11.7) | <0.001 |
| Tying | (SpO2) | 94.6 (2.3) | 93.5 (3.0) | 0.022 |
| (PR) | 179.6 (20.1) | 192.6 (15.7) | <0.001 |
| Excision | (SpO2) | 95.9 (1.9) | 94.8 (3.4) | 0.071 |
| (PR) | 161.5 (16.9) | 178.2 (15.5) | <0.001 |
p<0.05 is statistically significant
The mean NIPS were significantly higher in the placebo group compared to the oral ketamine group in all stages of the procedure (p-values <0.001, respectively) [Table/Fig-4].
Comparison of mean intra-procedural NIPS scores.
| Stage of procedure | Group A Mean (SD) | Group B Mean (SD) | p-value (t-test) |
|---|
| Adhesiolysis | 3.8 (1.8) | 4.9 (0.3) | <0.001 |
| Dorsal slit | 3.8 (1.7) | 4.9 (0.3) | <0.001 |
| Tying | 4.4 (1.3) | 4.9 (0.8) | 0.001 |
| Excision | 3.7 (1.5) | 4.8 (0.4) | <0.001 |
| Overall mean | 3.93 (1.58) | 4.88 (0.45) | <0.001 |
p<0.05 is statistically significant
There was no significant difference in the occurrence of side-effects between the two groups. The side-effects were all self-limiting, and neonates with excessive salivation were cleaned and re-positioned with satisfactory results [Table/Fig-5].
Comparison of occurence of side-effects.
| Side effects | Group A (n=61) | Group B (n=60) | p-value Fisher’s-exact test |
|---|
| Increased salivation | 5 | 1 | 0.061 |
| Vomiting | 1 | 0 |
| Hiccup | 1 | 0 |
| Sub-total | 7 | 1 |
Discussion
The study revealed that orally administered ketamine produced a significant intraoperative analgesia during neonatal circumcision when compared to a placebo. Majority of the procedures were carried out in neonates around the 15th day of life. This was on account of the traditional timing of neonatal circumcision in the environment. The traditional thinking was that the timing of the procedures is best from the 8th day and very importantly after the umbilical cord has fallen off [25]. This also was reflected in the average weight of the neonates of 3.67 kg and 3.65 kg. When the initial reduction in weight that occurs in the newborn, due to fluid shift is taken into consideration, it puts these values into a proper perspective.
With an average SpO2 and PRs above 96% and 140 for both groups [Table/Fig-2], the neonates were fairly stable and comparable for the procedure. In this study, neonates that received oral ketamine had a significantly higher SpO2 during the tying stage of the circumcision. This shows that oral ketamine provided significant analgesia for the procedure. This is significant considering that the tying is the most painful stage. Pain reduces the blood arterial oxygen saturation which is reflected in the SpO2 measurements [10]. The precise mechanism for the reduction of blood oxygen saturation by pain may be due to breath hold, hypoventilation, among others during crying. The analgesic effect provided by oral ketamine during the plastibell circumcision ensured that the blood oxygen saturation is higher in the neonates of group A (oral ketamine), than of group B (placebo). It means less stress and therefore safety for those neonates that received oral ketamine [10,12].
The mean PRs were lower at all the stages of the procedure in the oral ketamine group relative to the placebo group. These were significant in the dorsal slit, tying and excision stages. Pain increases the heart rate and hence PR. This suggests that the neonates that received oral ketamine syrup felt less pain relative to the neonates who received placebo. It suggests also that oral ketamine provided analgesia during the procedure. Pain is a stressor that stimulates increased cardiac activity, among other metabolic responses, with its attendant demands on the body. Neonates, that received oral ketamine mount less metabolic response to trauma and are therefore, safer during and after the procedure [9-12].
PRs readings at the 3 stages of dorsal slit, tying, and excision significantly reflected the analgesic properties of oral ketamine over placebo, while the SpO2 reading did so in only stage (tying). The differences in the measurements of SpO2 and PR could be attributed to the workings of the pulse oximeter. There are variations in sensitivity of pulse oximeter. The manufacturers alluded that it may take a maximum of five seconds for a change in SpO2 reading to reflect. These may mean that PR readings are more sensitivity than SpO2 within the procedure. This is coupled with the fact that there were no observed strict time-intervals between the stages in order to take readings. All these may have compromised the accuracy and sensitivity of the SpO2 readings.
The NIPS scores were quite informative. The mean NIPS score for the oral ketamine group were less than the placebo group in all the stages and were statistically significant in all. This portrays the capacity of oral ketamine to provide analgesia. The sedative effect of oral ketamine may also have contributed to the low NIPS score of the group. The maximum score of the scale is 7. The mean NIPS score for the placebo ranged from 4.8 to 4.9, approaching the maximum score of 5. The mean NIPS score for the oral ketamine group in all stages ranged from 3.7 to 4.4. The implication was that oral ketamine besides its analgesic effect appeal to the sense of the “modern mother” and doctor who do not want the neonates to cry out in pain while undergoing the procedure.
Oral ketamine has been shown to provide a measure of analgesia for those in acute and chronic pain [16,17,19,21]. Sareenmma E et al., found that ketamine has no effect in attenuating pain associated with suctioning in newborn infants on ventilator [21]. They used heart rate, arterial blood pressure and pain scoring systems of Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) and NIPS. However, they used intravenous ketamine in doses ranging from 0.5 mg/kg to 2 mg/kg. Ketamine administered orally is converted to nor-ketamine (four-fifth) by the GIT, with minimal unaltered ketamine (16%) entering the system. The nor-ketamine is thought to produce the major analgesic effect of ketamine. Parenterally administered ketamine will produce lesser analgesic effect for procedural pain, compared to orally administered ketamine. Ogboli-Nwasor E et al., found that oral ketamine is very effective in the emergency room as analgesia for fracture manipulations [19]. This was similar to the findings in another study by Buvanendran A et al., [26]. The study by Ogboli-Nwasor E et al., was among adults and soda water was used as an elixir to sweeten the ketamine [19]. This study was on neonates and simple sucrose was used to make ketamine syrup.
In this study, seven out of 61 neonates in the oral ketamine group had complications, while in the placebo group one out of 60 neonates had complications. This was statistically not significant. The complications noted in the oral ketamine group were increased salivation, nonbilous vomiting and hiccough. These were handled by cleaning and repositioning. Studies on ketamine usage had recommended using anti-sialogogue [27]. This was not used in this study as ketamine administered orally delivers more of norketamine into the system. Moreover, the neonates were not anaesthetised and their swallowing reflexes were not altered, hence they were able to swallow their saliva [16]. In the placebo group, one patient presented with increased salivation during the procedure, and this was handled in a similar fashion like the oral ketamine group. The cause of increased salivation in this group was not clear, but may be from pain. These adverse effects did not continue in the immediate post-procedural period.
Limitation(s)
Neonates are conversant with the mother’s body and cuddling. When separated from such, they may cry or become restless. These may influence the outcome measures. Attempts were made to eliminate them. However, it was not possible to do so completely. Again the overlapping effect of the pains in one stage to the next stage could not be averted during the procedure. These were limitations of this study.
Conclusion(s)
Oral ketamine provides effective and safe analgesia for neonatal Plastibell® circumcision, in comparison with placebo. It is recommended for use in neonatal circumcision. It is suggested that studies be conducted for its use in other procedures and in other age groups. Further study is necessary to compare the efficacy of oral ketamine and other forms of procedural analgesia during neonatal circumcision in order to draw better conclusion and to properly place oral ketamine in its rank among other analgesic agents.
p<0.05 is statistically significant
p<0.05 is statistically significant
p<0.05 is statistically significant
[1]. Lander J, Brady-Fryer B, Metcalfe JB, Nazarali S, Muttitt S, Comparison of ring block, dorsal penile nerve, and topical anaesthesia for neonatal circumcision: A randomised controlled trialJAMA 1997 278:2157-62.10.1001/jama.1997.035502400470329417009 [Google Scholar] [CrossRef] [PubMed]
[2]. Howard CR, Weitzman ML, Howard FM, Acetaminophen analgesia in neonatal circumcision: The effect on painPaediatrics 1994 93:641-46.10.1542/peds.93.4.641 [Google Scholar] [CrossRef]
[3]. Brady-Fryer B, Wiebe N, Lander JA, Pain relief for neonatal circumcisionThe Cochrane library; Cochrane neonatal group 2005 John Wiley and Sons Ltd10.1002/14651858.CD004217.pub215495086 [Google Scholar] [CrossRef] [PubMed]
[4]. Modekwe VI, Ugwu JO, Ekwunife OH, Osuigwe AN, Obiechina SO, Okpalike IV, Comparison of the efficacy of eutectic mixture of local anesthetics (EMLA) and dorsal penile nerve block (DPNB) in neonatal circumcisionNiger J Clin Pract 2019 22:1737-41.10.4103/njcp.njcp_266_1931793482 [Google Scholar] [CrossRef] [PubMed]
[5]. Devine CJ, Jordan GH, Surgery of the penis and urethra. In Walsh PC, Retik AB, Stamey TA, Vaughan ED (eds)Campbells Urology. (6th Ed) 1992 Vol 3Philadelphia USASaunder:2964 [Google Scholar]
[6]. Guyton AC, Hall JE, Somatic sensations II: pain, headache, and thermal sensation. In: Guyton and Hall (eds)Textbook of Medical Physiology 2006 11th EdPhiladelphia, Pennsylvania, USAElsevier Saunders:598-606. [Google Scholar]
[7]. Anand KJS, and the international evidence-based group for neonatal painConsensus statement for the prevention and management of pain in the newbornArch Pediatr Adolesc Med 2001 155:173-80.10.1001/archpedi.155.2.17311177093 [Google Scholar] [CrossRef] [PubMed]
[8]. Taddio A, Katz J, The effects of early pain experience in neonates on pain responses in infancy and childhoodPediatr-Drugs 2005 7:245-57.https://doi.org/10.2165/00148581-200507040-0000410.2165/00148581-200507040-0000416117561 [Google Scholar] [CrossRef] [PubMed]
[9]. Talbert LM, Kraybill EN, Potter HD, Adrenal cortical response to circumcision in the neonateObstet Gynecol 1976 48:208-10. [Google Scholar]
[10]. Stevens BJ, Franck LS, Assessment and management of pain in neonatesPaediatric Drugs 2001 3:539-58.https://doi.org/10.2165/00128072-200103070-0000410.2165/00128072-200103070-0000411513283 [Google Scholar] [CrossRef] [PubMed]
[11]. Anders TF, Chalemian RJ, The effects of circumcision on sleep-wake states in human neonatesPsychosomatic Medicine 1974 36:174-79.10.1097/00006842-197403000-000094360754 [Google Scholar] [CrossRef] [PubMed]
[12]. Weisman SJ, Bernstein B, Schecter NL, Consequences of inadequate analgesia during painful procedures in childrenArch Pediatr Adolesc Med 1998 152:147-49.10.1001/archpedi.152.2.1479491040 [Google Scholar] [CrossRef] [PubMed]
[13]. Taddio A, Chambers TC, Halperin SA, Ipp M, Lockett D, Rieder MJ, Inadequate pain management during routine childhood immunizations: The nerve of itClinical Therapeutics 2009 31:S152-67.https://doi.org/10.1016/j.clinthera.2009.07.02210.1016/j.clinthera.2009.07.02219781434 [Google Scholar] [CrossRef] [PubMed]
[14]. American Academy of Pediatrics, Committee on Fetus and Newborn and Section on Surgery, Section on Anesthesiology and Pain Medicine, Canadian Paediatric Society and Fetus and Newborn CommitteePrevention and Management of Pain in the Neonate: An UpdatePediatrics 2006 118:2231-41.10.1542/peds.2006-227717079598 [Google Scholar] [CrossRef] [PubMed]
[15]. Jian X, Hong L, Ketamine-An Update on Its Clinical Uses and AbusesCNS Neurosci Ther 2014 20:1015-20.10.1111/cns.1236325417928 [Google Scholar] [CrossRef] [PubMed]
[16]. Craven R, KetamineAnaesthesia 2007 62:48-53.10.1111/j.1365-2044.2007.05298.x17937714 [Google Scholar] [CrossRef] [PubMed]
[17]. Brunette KEJ, Anderson BJ, Thomas J, Wiesner L, Herd DW, Schulein S, Exploring the pharmacokinetics of oral ketamine in children undergoing burns proceduresPediatric Anesthesia 2011 21:653-62.10.1111/j.1460-9592.2011.03548.x21355949 [Google Scholar] [CrossRef] [PubMed]
[18]. Barkan S, Breitbart R, Brenner-Zada G, Feldon M, Assa A, Toledano M, A double-blind, randomised, placebo-controlled trial of oral midazolam plus oral ketamine for sedation of children during laceration repairEmerg Med J 2014 31:649-53.10.1136/emermed-2012-20218923686730 [Google Scholar] [CrossRef] [PubMed]
[19]. Ogboli-Nwasor EO, Amaefule KE, Audu SS, Use of oral ketamine for analgesia during reduction/manipulation of fracture/dislocation in the emergency room: An initial experience in a low resource settingPain Studies and Treatment 2014 2:17-20.10.4236/pst.2014.21004 [Google Scholar] [CrossRef]
[20]. Funk W, Jakob W, Riedl T, Taeger K, Oral preanaesthetic medication for children: Double-blind randomised study of a combination of midazolam and ketamine vs midazolam or ketamine aloneBritish Journal of Anaesthesia 2000 85:335-40.10.1093/oxfordjournals.bja.a01343510793592 [Google Scholar] [CrossRef] [PubMed]
[21]. Saarenmaaa E, Neuvonenb PJ, Huttunenc P, Fellmana V, Ketamine for procedural pain relief in newborn infantsArch Dis Child Fetal Neonatal Ed 2001 85:F53-F56.10.1136/fn.85.1.F5311420324 [Google Scholar] [CrossRef] [PubMed]
[22]. Okeke LI, Asinobi AA, Ikuerowo OS, Epidemiology of complications of male circumcision in Ibadan, NigeriaBMC Urology 2006 6:2110.1186/1471-2490-6-2116934157 [Google Scholar] [CrossRef] [PubMed]
[23]. Sowande OA, Adejuyigbe O, Circumcision mishaps: A continuing challenge in the developing countriesEast and Central African Journal of Surgery 2009 14:109-13.10.4103/0189-6725.4856919661659 [Google Scholar] [CrossRef] [PubMed]
[24]. Abdur-Rahman LO, Musa OI, Oshagbemi GO, Community-based study of circumcision practices in NigeriaAnnals of Tropical Medicine and Public Health 2012 5:231-35.10.4103/1755-6783.98625 [Google Scholar] [CrossRef]
[25]. Ekwunife OH, Ugwu JO, Okoli CC, Modekwe VI, Osuigwe AN, Parental circumcision preferences and early outcome of plastibell circumcision in a Nigerian tertiary hospitalAfr J Paediatr Surg 2015 12:251-56.10.4103/0189-6725.17256526712290 [Google Scholar] [CrossRef] [PubMed]
[26]. Buvanendran A, Kroin JS, Rajagopal A, Robison SJ, Moric M, Tuman KJ, Oral ketamine for acute pain management after amputation surgeryPain Medicine 2018 19:1265-70.https://doi.org/10.1093/pm/pnx22910.1093/pm/pnx22929025089 [Google Scholar] [CrossRef] [PubMed]
[27]. Melendez E, Bachur R, Serious adverse events during procedural sedation with ketaminePediatric Emergency Care 2009 25:325-28.10.1097/PEC.0b013e3181a341e019404223 [Google Scholar] [CrossRef] [PubMed]