Celiac disease is a chronic small bowel enteropathy with an underlying autoimmune mechanism precipitated by exposure to dietary gluten in genetically predisposed people. Celiac specific antibodies comprise of auto-antibodies against tTG including Endomysial Antibodies (EMA) and antibodies against deaminated forms of gliadin peptides. The prevalence of celiac disease varies in different parts of the world. In the western countries, it ranges from 0.7% to as high as 2%. In India, most of the cases are reported from the northern parts with an average prevalence of 1% [1]. It is often cited as the most common cause of chronic diarrhoea in children which accounts for 26% and 56% of chronic diarrhoea among children and adults in Western and Northern India, respectively [1]. The silent celiac disease is approximately seven times more common than symptomatic disease worldwide, and atypical presentation occurs in 30-40% of patients diagnosed with celiac disease in India [2]. Thus, the numbers represent only the tip of the iceberg and the actual burden is much more. The ratio of diagnosed to undiagnosed cases however varies widely from country-to-country [3-5].
In the recent days, due to increased awareness and recognition of atypical non-gastrointestinal manifestations like anaemia, short stature etc., with widespread use of serology for screening, more and more celiac cases are being identified especially among children. Some experts also attribute it to the fact that modern hexaploid wheat (triticum aestivum) is more antigenic than the ancient diploid wheat (triticummonoccum). Hence, present study was planned to estimate the prevalence of celiac disease in children with chronic diarrhoea attending a tertiary care centre.
Materials and Methods
This cross-sectional study was conducted in the Department of Paediatrics, PGIMER, Dr. RML Hospital, New Delhi, India, from November 2015 to January 2018, after taking ethical clearance from the Institutional Ethical Committee (IEC no. 4903). Sample size required was estimated to be 890 subjects based on an assumed prevalence of celiac disease to be 1% in northern Indian population [6]. Informed written consent was taken from all the participants’ parents/guardians before the enrollment.
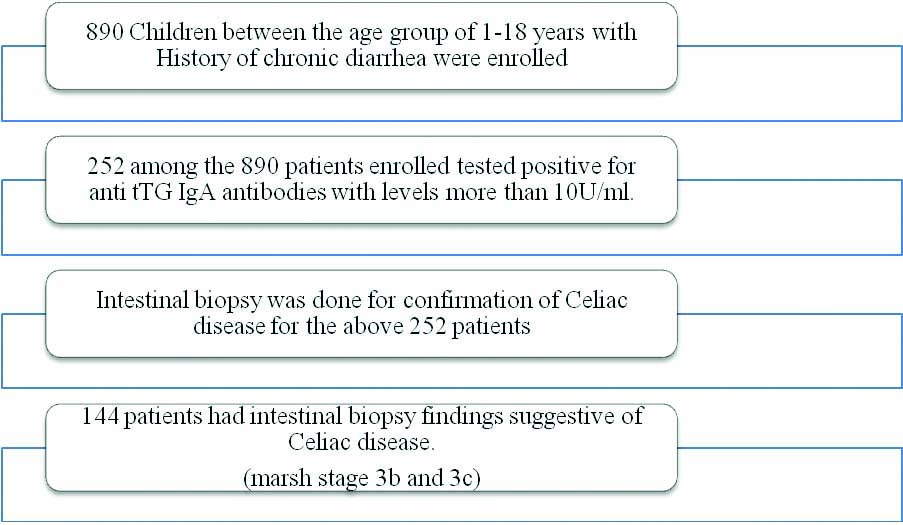
Inclusion and Exclusion criteria: A total of 890 children with history of chronic diarrhoea (between the age group of 1-18 years), were enrolled from the paediatric gastroenterology clinic, paediatric outpatient department and wards [Table/Fig-1]. Children with history of any previous hospitalisations lasting more than two weeks, any abdominal surgeries or trauma or a known chronic inflammatory disorder were excluded from the study.
Flow chart depicting patient enrollment.
For the purpose of the study, chronic diarrhoea was defined as increased frequency or liquidity of the stools lasting for more than two weeks with or without treatment [1]. Children were screened with serological tests and in those who tested positive serology; the diagnosis was confirmed by intestinal (duodenal) biopsy. The definition of the Celiac disease was based on the guidelines by World Gastroenterology Organisation -Guidelines for Celiac Disease 2012 for Children [7]. It is as follows-Children with clinical symptoms or asymptomatic children with family history with positive serology (IgA (Immunoglobulin A) anti tTG antibodies) and biopsy findings (evidence of villous atrophy– Marsh staging 3b,c).
Microscopic findings were categorised according to Modified Marsh Grading. Marsh 3b i.e., subtotal villous atrophy with atrophic but recognisable villi and Marsh 3c with total villous atrophy with absent villi were diagnostic of celiac disease [8,9].
Statistical Analysis
Statistical analysis was performed by the Statistical Package for the Social Sciences (SPSS) program for Windows, version 17.0. Continuous variables are presented as mean±SD (Standard Deviation) and categorical variables are presented as absolute numbers and percentage.
Results
Of the 890 patients with chronic diarrhoea enrolled in the study, 356 patients were males and 534 patients were females [Table/Fig-2]. Mean age of the enrolled patients was 7.72±3.26 years. Out of 890 patients with chronic diarrhoea, 88 patients had history of blood in stools, 270 patients had history of fever, 256 patients had associated vomiting, 377 patients had abdominal pain and 34 patients had abdominal distension at enrollment. Amongst the patients, 346 (38.9%) had wasting and 445 (50%) had stunting i.e., weight and height less than 3rd percentile, 99 (11.1%) of the patients had both wasting and stunting.
General characteristics of study population (N=890).
| Age distribution (years) | |
|---|
| <5 | 36.0% |
| 6-9 | 27.8% |
| 10-12 | 30.6% |
| >12 | 5.6% |
| Mean age | 7.72±3.26 years |
| Male:female ratio | 1:0.8 |
| Mean haemoglobin (g%) | 8.71±2.51 |
| Mean serum sodium (mmol/L) | 138.64±4.02 |
| Mean serum potassium (mmol/L) | 4.55±0.76 |
| Mean blood urea (mg/dL) | 20.85±7.57 |
| Mean serum creatinine (mg/dL) | 0.48±0.19 |
| Mean serum albumin (g/dL) | 3.05±0.59 |
Total 252 among the 890 patients enrolled tested positive for anti tTG IgA antibodies with levels more than 10U/ml [1]. Among the 252 patients with positive serology, intestinal biopsy was done for confirmation and 144 patients had intestinal biopsy findings suggestive of Celiac disease. Among them, 93 patients had Marsh stage 3b and 51 patients had Marsh stage 3c changes. Thus, the hospital prevalence was found to be 16.17% among children with chronic diarrhoea. The age distribution is shown in [Table/Fig-2].
Discussion
Of the 890 patients with chronic diarrhoea, 252 patients were tested positive for anti-tTG antibodies with levels more than 10 U/mL. That is, the serological positivity for celiac disease among children with chronic diarrhoea was 28.3%. Among the 252 patients with positive serology, 144 patients had intestinal biopsy findings suggesting Marsh stage 3b and 3c changes, confirming the diagnosis of celiac disease. Thus, the hospital prevalence was found to be 16.17% among children with chronic diarrhoea.
More than one third of the cases (36.0%) were aged less than 5 years with a mean age of 7.72±3.26 years. Previous Indian studies have reported similar age at diagnosis for celiac patients. Mohindra S et al., reported a mean age at diagnosis of 2.4 (range 0.5-10) years and 8.3 (3-14) years [10]. Similarly, Sharma A et al., and Agarwal N et al., studying atypical/non-diarrhoeal celiac disease reported a slightly higher mean age at diagnosis of 10.4 years and 10.2 years respectively [2,11].
In a similar study by Bengi G et al., 79 patients of Inflammatory Bowel Disease (IBD) were evaluated for celiac disease and 5.06% (n=4) tested positive [12]. In a similar study, by Eskander A et al., 101 patients with IBD were screened for celiac disease where 10 patients (9.9%) had positive serology based on IgA-tTG antibodies, three (approximately 3%) had celiac disease based on biopsy findings [13].
In another study by Tursi A et al., which included 27 patients affected by Crohn’s disease, celiac antibody tests were positive in 11 out of 27 patients [14]. Nine out of them had signs of duodenal endoscopic damage, and five of nine had histologic features of celiac 18.52% disease (of overall CD population studied). In this study, similar results were obtained with prevalence of biopsy diagnosed celiac disease to be 16.1% among children with chronic diarrhoea more than two weeks.
Limitation(s)
In this study, the children were tested for Ig-A anti-tTG antibodies only and hence children with IgA deficiency would be wrongly diagnosed to be negative for celiac disease. As the diagnosis required intestinal changes suggestive of marsh grade 3b or 3c, children with early stages of the disease were also falsely excluded and the same were not followed-up with repeat intestinal biopsy. As it was a cross-sectional study, detailed evaluation of all the children for other causes of chronic diarrhoea could not be done.
Conclusion(s)
Celiac disease is one of the important causes of chronic diarrhoea especially in children presenting to a tertiary care centre, but the diagnosed cases represent only the tip of the iceberg. Hence, it is important to actively look for the diagnosis with high index of suspicion in such children especially because the treatment involves a major change in lifestyle.