Whipple’s PD was originally used in the treatment of pancreatic periampullary neoplasia. Allessandro Codivilla, an Italian surgeon is credited with having performed the first PD in 1898 for a patient with a lesion in the head of pancreas. His patient survived for only 24 days [1]. A year later in 1899 William Halsted, one of the great contributors to modern surgery, presented the first successful resection of ampullary cancer in which he excised a portion of the duodenum [2]. In 1909, Walter Carol Kausch in Berlin performed a successful staged PD in a patient with periampullary tumour. This patient survived for nine months [3]. Brunschwig was the first to use the operation successfully for the cancer of the head of pancreas in 1937. In 1935, Dr Allen, Oldfather Whipple reported three cases of two staged PD, of which in one case the patient underwent total duodenectomy. In 1940, Whipple’s surgery became an one stage procedure. Whipple AO performed a total of 37 pancreatico-duodenectomies over his career of which 30 were performed for periampullary carcinomas and seven for chronic pancreatitis [4]. He proposed various modifications to the original one stage procedure which significantly improved the mortality associated with this surgery [5]. Thus, PD was performed many years earlier than the description published by Whipple and colleagues.
The use of this procedure is increasing and carried out in many tertiary centres with better patient survival outcomes. Due to the complexity of the specimen, a certain level of expertise is required in gross assessment since the tumour origin, margins and staging of the tumour has prognostic implications. This study was undertaken with an aim to study the Whipple’s specimens, analyse the clinicopathological parameters and stage the tumours according to the American Joint Committee on Cancer (AJCC) guidelines [6].
Materials and Methods
This was an observational retrospective time bound descriptive study done in the Department of Pathology, Father Muller Medical College Hospital, Mangalore, Karnataka with a primary objective to histopathologically categorise, grade and stage the tumour as per AJCC-TNM (Tumour-Node-Metastasis). The study was undertaken after obtaining an Ethical Clearance from the Hospital Ethical Committee (FMMCIEC/CCM/292/2019).
Inclusion criteria: Those Whipple’s specimens which had complete requisition form with adequately filled clinicodemographical data and were received in the Department of Histopathology between May 2014 to April 2019 were included in the study.
Exclusion criteria: The specimens with prior history of neoadjuvant treatment were excluded from the study.
Study Procedure
Histopathological and clinicodemographical data of all the patients who underwent Whipple’s procedure were retrieved from the Medical Records Department and analysed. The specimens were also retrieved from the departmental museum and grossing protocols were analysed from the records for the type of grossing method used and adequacy of margin status. In view of rarity of PD specimens, a thorough understanding of the anatomy and grossing aspects was undertaken.
Strict criteria were applied for categorisation of the tumours as ampullary, periampullary, mixed ampullary periampullary, common bile duct and duodenal [6-11]-
Tumours with epicentre in the ampulla were categorised as ampullary carcinomas.
Tumours growing circumferentially around the ampulla were categorised as periampullary carcinomas.
Tumours with both ampullary and periampullary growth pattern were categorised as mixed ampullary/periampullary carcinomas.
Tumours involving the circumference of the common bile duct were categorised as common bile duct tumours.
Tumours with epicentre in the duodenal wall exhibiting thickening or ulceroproliferative growth protruding into the lumen were labelled as duodenal carcinomas.
The H&E slides were retrieved and reviewed by two pathologists for histopathological categorisation and grading, origin of the tumour, lymphovascular and perineural invasion, margin and lymph node status were assessed. The margins assessed were pancreatic neck margin, cystic duct margin, uncinate margin, superior mesenteric vein/portal vein and gastric/duodenal margins. Sections from anterior and posterior surface of the pancreas were also studied. Consensus was reached upon in all the cases. Cytochemical stains for mucin detection like Mucicarmine and Alcian blue were employed wherever required. CD117 (ckit) was done for one of the cases. TNM staging of the tumour was done based on AJCC TNM classification [6].
Statistical Analysis
Simple descriptive statistical analysis was done by calculating Percentage, Mean, median and Range. The p-value was calculated using Fischer-exact test (SPSS software version 2) and value of <0.05 was considered statistically significant.
Results
Demographic Results
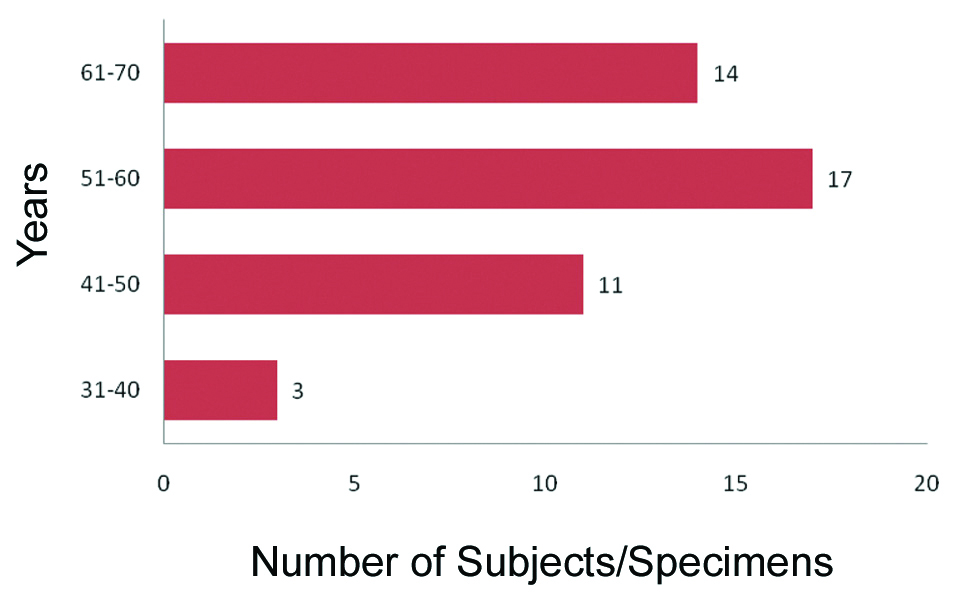
A total of 45 patients underwent Whipple’s procedure, of which 28 (62.2%) were males and 17 (37.8%) were females with a ratio being 1.6:1. Out of total 45 Whipple’s specimen, a classic Whipple’s procedure specimen was observed in 40 cases (88.8%). Pylorus preserving Whipple’s was seen in the remaining five cases (11.2%). Majority of the patients were in the 5th and 6th decade, mean age being 56.2 years [Table/Fig-1]. The age range was 33 to 70 years. The median age for periampullary, ampullary and pancreatic tumours were 50, 60 and 56.5 years, respectively.
Age distribution in Whipple’s PD.
PD: Pancreatic duodenectomy
Clinicopathological Results
The most common symptom was jaundice (33 cases-73.3%) followed by pain abdomen (7 cases-15.6%). The mean tumour size was 2.8 cm [Table/Fig-2]. Malignancy was seen in (43 cases-95.6%). Remaining two were inflammatory lesions (2.2%). The most common site of localisation of tumours was periampullary (16 cases-37.2%) followed by pancreatic head (15 cases-34.9%). In three cases, there was involvement of periampullary as well as ampullary region and the exact origin of tumour could not be derived upon. Hence, these tumours were categorised as mixed (ampullary and periampullary). The most common histological subtype was adenocarcinoma (40 cases-93.02%), of which majority were of the intestinal type (38 cases-95%), remaining were that of pancreatico-biliary type (2 cases-5%). Out of 40 adenocarcinomas, well-differentiated grade was seen in 28 cases (70%). There were two cases of neuroendocrine tumours (2 cases-4.7%) and a single case of malignant GIST (1 case-2.3%). The remaining two cases were that of chronic pancreatitis. Lymphovascular invasion were seen in 16 cases (37.2%) and perineural invasion in 23 cases (53.5%). Lymph node metastasis was seen in 16 cases (37.2%). Margins were clear in most of the cases (37 cases-86.04%). T2 was the most common stage (16/43- 37.2%), followed by T3 (12/43-27.9%).T1 was seen in 11 out of 43 cases (25.6%) and T4 was seen least (4/43-9.3%). Additional findings were seen in two cases of, one being duodenal GIST and the other being pancreatic heterotopia in the duodenum [Table/Fig-3].
Size distribution of the lesions in Whipple’s PD.
| Location | <1 cm | 1.1-2 cm | 2.1-3 cm | 3.1-4 cm | 4.1-5 cm | >5 cm |
|---|
| Ampullary carcinoma (n=7) | 1 | 5 | - | 1 | - | - |
| Periampullary carcinoma (n=16) | 2 | 7 | 2 | 2 | 3 | - |
| Mixed (ampullary+periampullary) (n=3) | - | - | - | 3 | | |
| Pancreatic head (n=15) | - | 5 | 3 | 4 | - | 3 |
| Duodenum (n=2) | - | - | 1 | - | - | 1 |
Table 2 has only 43 cases because 2 cases were that of chronic pancreatitis without any lesion/tumor per say
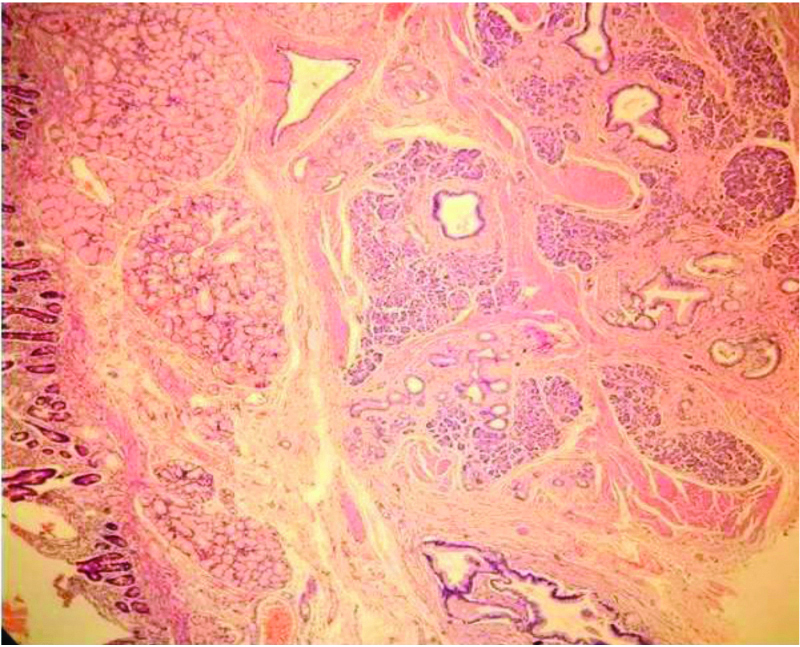
Pancreatic heterotopia in the duodenum additional findings (100X H&E).
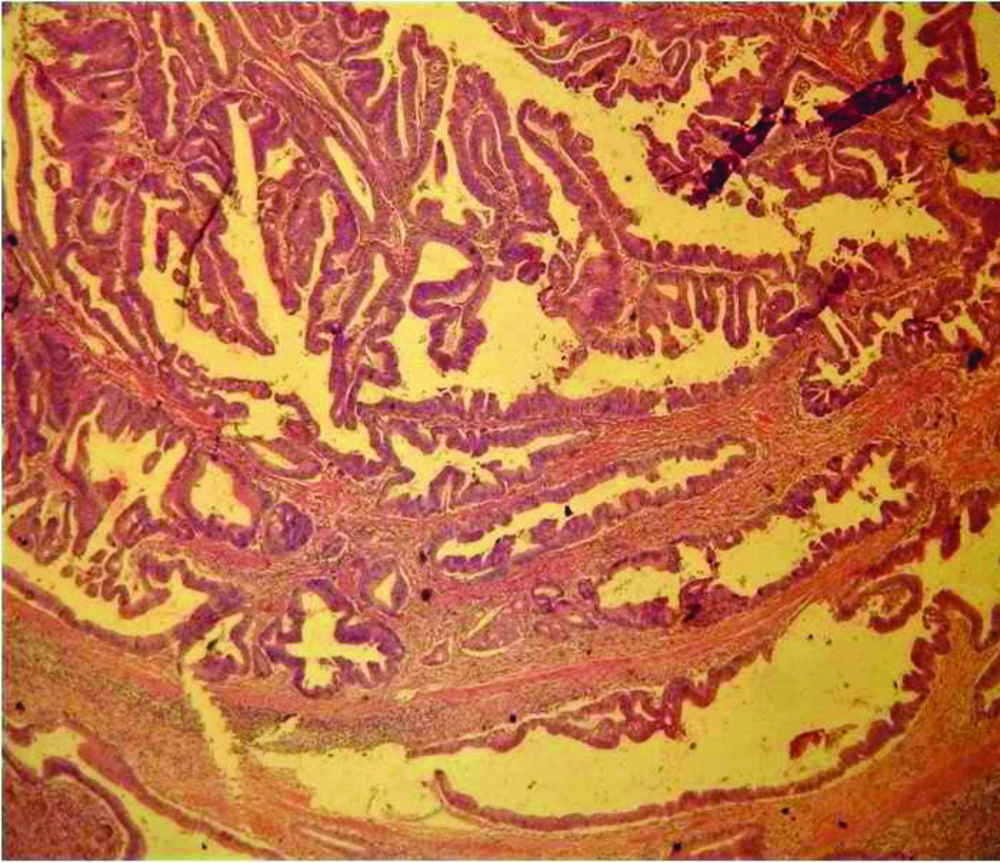
Periampullary Cancers
Majority of the tumours in this category were seen in the size range of 1.1-2 cm. Most common were well differentiated tumours (14/16 cases-87.5%), the remaining were moderately differentiated. All the adenocarcinomas were of intestinal type except for one case of pancreatico-biliary type. Three cases of periampullary cancers had positive lymph nodes. The lymph nodes dissected were in the range of 3 to 22. Most of the specimens had clear margins (15 cases-93.75%). T2 was the most common stage in this category (10/16 cases-62.5%).
Pancreatic Cancer
Of the 15 cases of this category, majority were of size range 1.1-2 cm (5 cases-33.3%) followed by 3.1-4 cm (4 cases-26.7%). Most common tumour category was adenocarcinoma in head of pancreas (13 cases 86.7%) followed by neuroendocrine tumour grade 2 (2 cases-13.3%) [Table/Fig-4]. Lymph node positivity was most commonly seen in pancreatic cancers (7 cases-46.7%) with a significant p-value of 0.001. Lymph nodes harvested in the range of 2-26. Lymphovascular invasion and perineural invasion were seen in 7 (p-value-0.494) and 11 cases (p-value<0.001), respectively [Table/Fig-5,6]. Margin positivity was seen in 4 cases (26.7%), of which 3 had a positive uncinate margin and one had both positive common bile duct margin as well as the pancreatic cut margin. In this category, T3 was the most common stage (6/15 cases-40%) [Table/Fig-7].
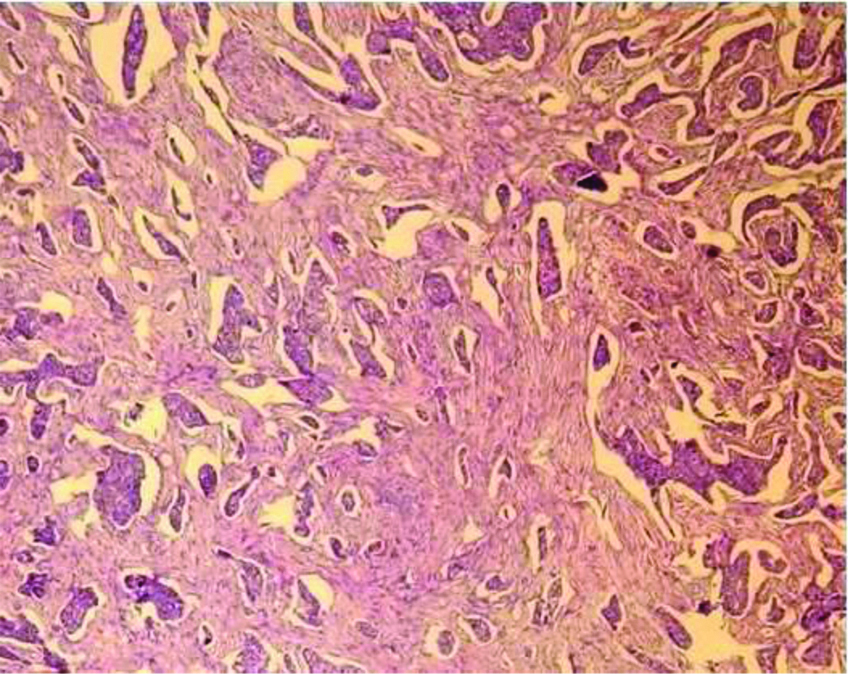
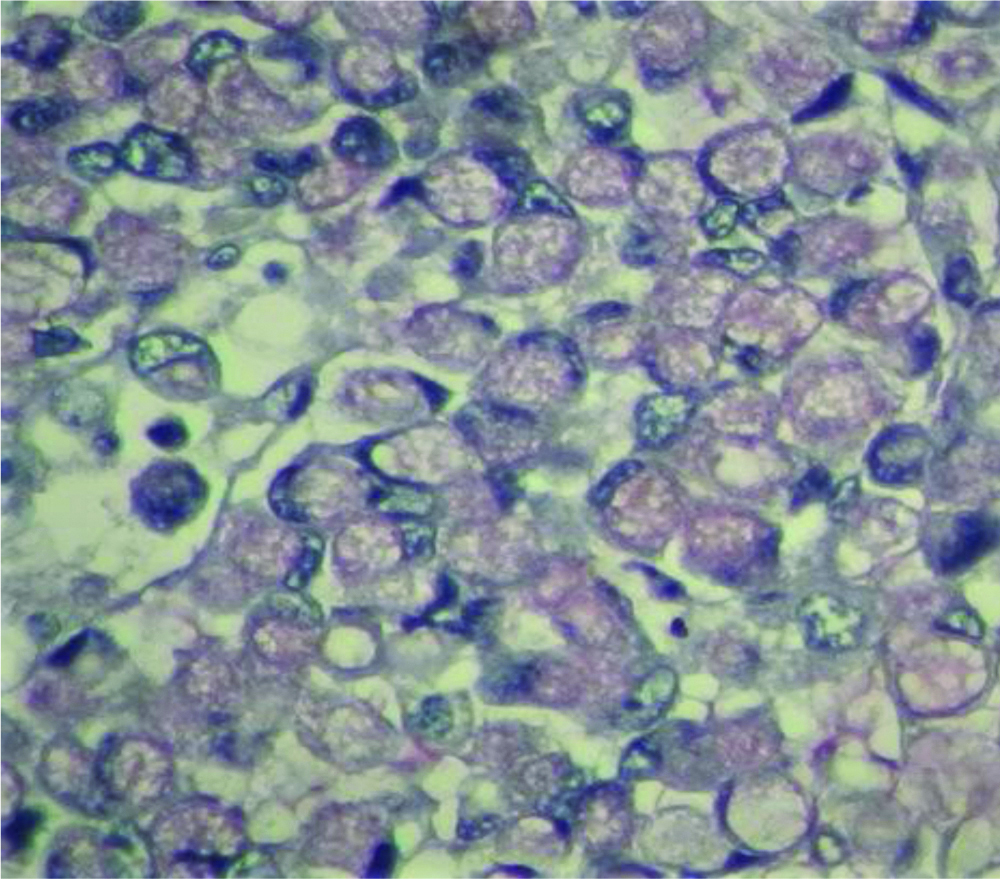
Neuroendocrine tumour Grade 2 (100X H&E).
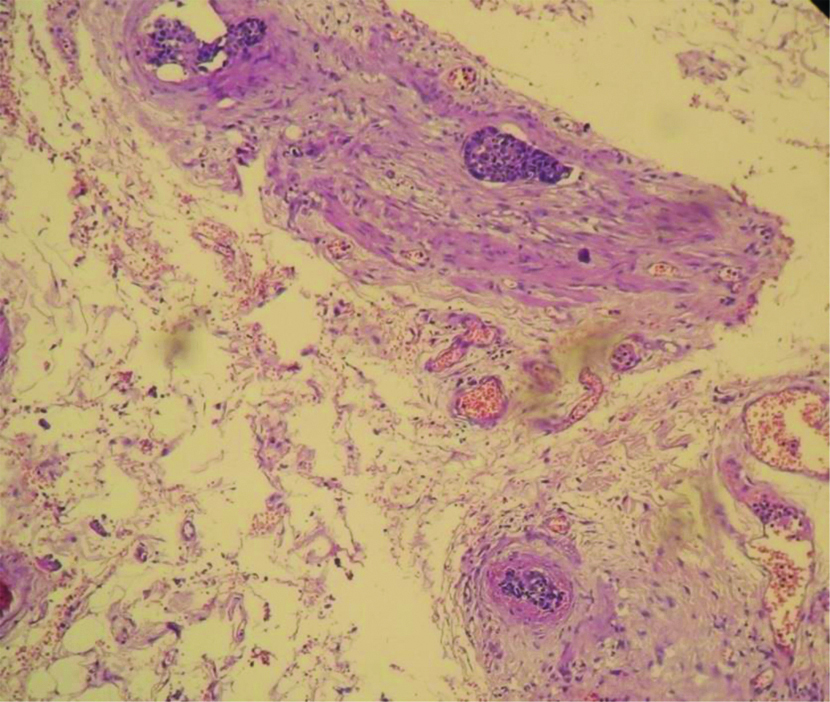
Angioinvasion seen in neuroendocrine tumour (100X H&E).
Neural invasion in adenocarcinoma head of pancreas (400X H&E).
Demographic and clinicopathological data.
| Characterstics of specimens | Periampullary (n=16) | Ampullary (n=7) | Mixed (periampullary and ampullary) (n=3) | Pancreatic (n=15) | Duodenal (n=2) | p-value |
|---|
| Median age (years-range) | 50 (33-68) | 57 (51-68) | 56 (51-70) | 53 (39-70) | 65.5 | 0.030 |
| Gender |
| Male (n) | 9 (56.2%) | 4 (57.1%) | 2 (66.7%) | 9 (60%) | 2 (100%) | |
| Female (n) | 7 (43.8%) | 3 (42.8%) | 1 (33.3%) | 6 (40%) | - | |
| Differentiation |
| n (%) for adenocarcinomaWellModeratePoor | 14 (87.5%)2 (12.5%)- | 4 (57.1%)1 (14.3%)2 (28.6%) | 2 (66.7%)1 (33.3%)- | 11 (84.6%)2 (15.4%)- | --1 (50%) | |
| OthersNeuroendocrine tumourMalignant GIST | - | - | - | 2 (13.3%)- | -1 (50%)# | |
| No of lymph nodes dissected (mean)Range | 9.13-22 | 13.65-25 | 810-32 | 52-26 | 8.55-12 | |
| Involved lymph nodes | 3 (18.8%) | 3 (42.9%) | 2 (66.7%) | 7 (46.7%) | 1 (50%) | |
| Lymphovascular invasion | 6 (37.5%) | 1 (14.3%) | 2 (66.7%) | 7 (46.7%) | - | 0.494 |
| Perineural invasion-present | 6 (37.5%) | 5 (71.4%) | 1 (33.3%) | 11 (73.3%) | - | <0.001 |
| Margin positivity | 1 (6.3%) | 1 (14.3%) | - | 4 (26.7%) | - | 0.108 |
#malignant GIST. Patients with pancreatic adenocarcinoma are more likely to have perineural invasion in comparision to ampullary/periampullary adenocarcinomas
p-value calculation done by Fischer-exact test with SPSS software version 2. p-value <0.05 to be considered significant. GIST: GastroIntestinal stromal tumors
Ampullary Cancers
Of the total 7 ampullary tumours, intestinal type of adenocarcinoma was seen in 6 cases with only one case with features of pancreatico-biliary type of adenocarcinoma [Table/Fig-8]. Well-differentiated tumours were common in this category (4/7 cases-57.1%) followed by poorly differentiated tumours (2/7 cases-28.8%) [Table/Fig-9,10]. The tumours were more commonly seen in the size group of 1.1-2 cm (5/7 cases-71.4%). Lymph node positivity were seen in 3 cases. Margin clearance was good in this category with only one case of margin positivity. Perineural invasion was seen in 5 cases (71.4%). Majority of the tumours were in T2 category (4/7 cases-57.1%).
Ampullary well-differentiated adenocarcinoma (100X H&E).
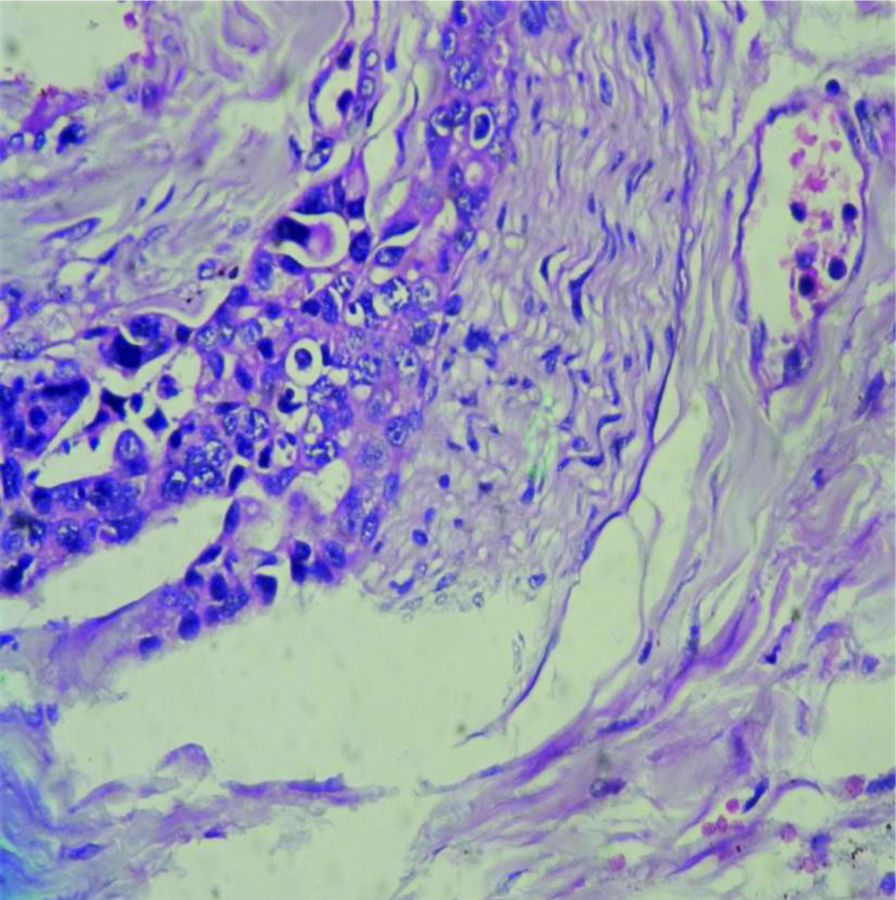
Well-differentiated ampullary adenocarcinoma-pancreaticobiliary type 100X H&E).
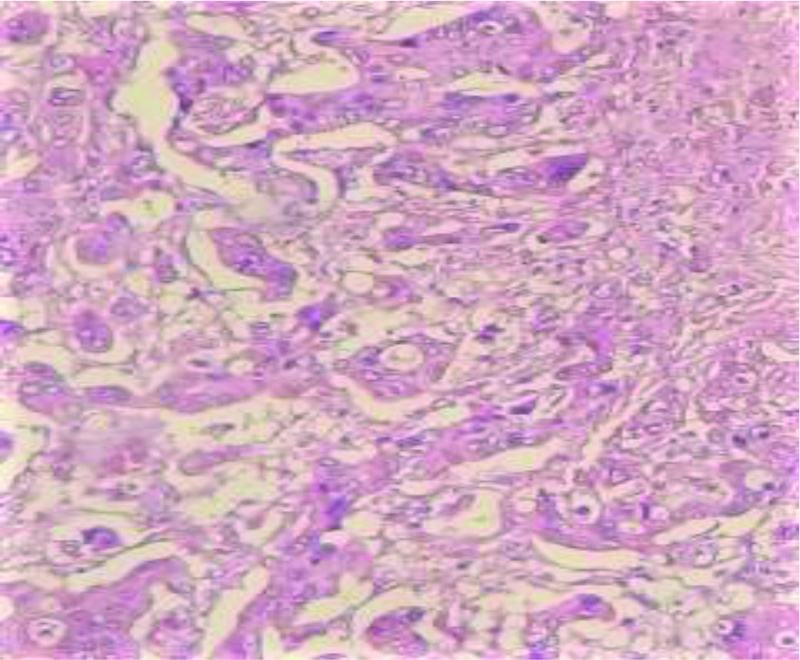
Mucin positivity in poorly differentiated ampullary carcinoma (Mucicarmine stain 400X H&E).
Mixed Tumours and Duodenal Tumours
All the mixed tumours measured 3.5 cm in the longest dimension. Two cases were of well-differentiated adenocarcinoma and one was moderately differentiated adenocarcinoma. All 3 cases were in the T2 category. Of the 2 cases of duodenal tumours one case was of poorly differentiated adenocarcinoma and the other was of malignant GIST which measured 14 cm in its longest dimension [Table/Fig-11,12]. Lymphovascular invasion, perineural invasion or margin positivity was not observed in the duodenal tumours.
Spindle shaped cells in malignant GIST (100X H&E).
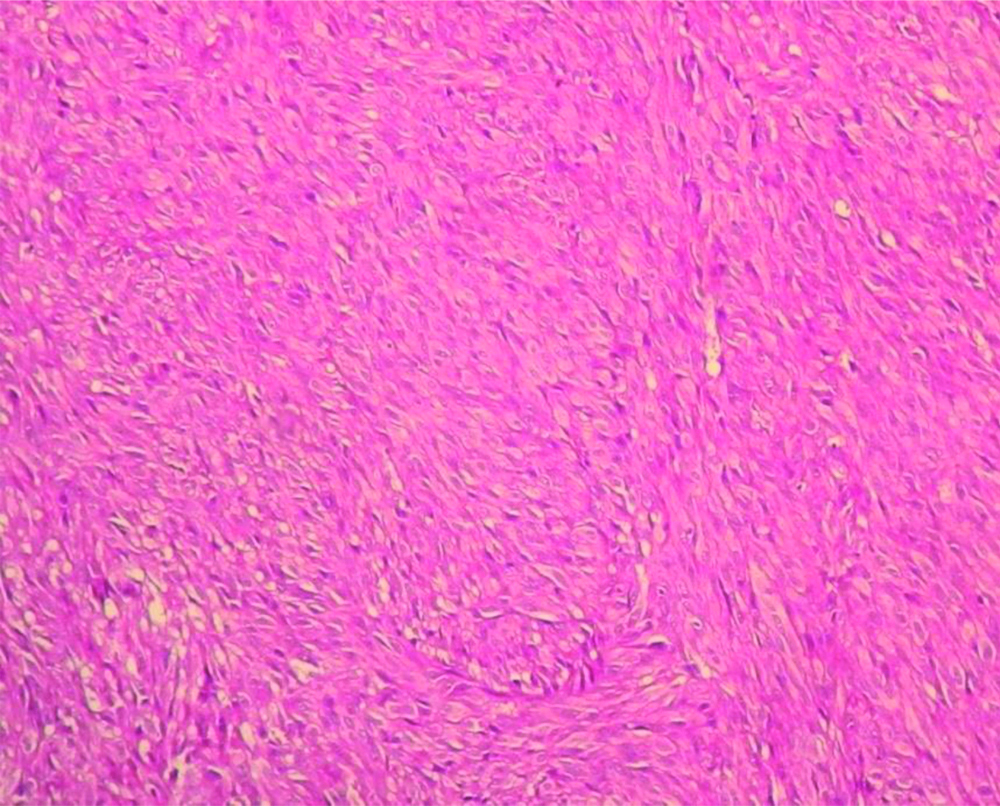
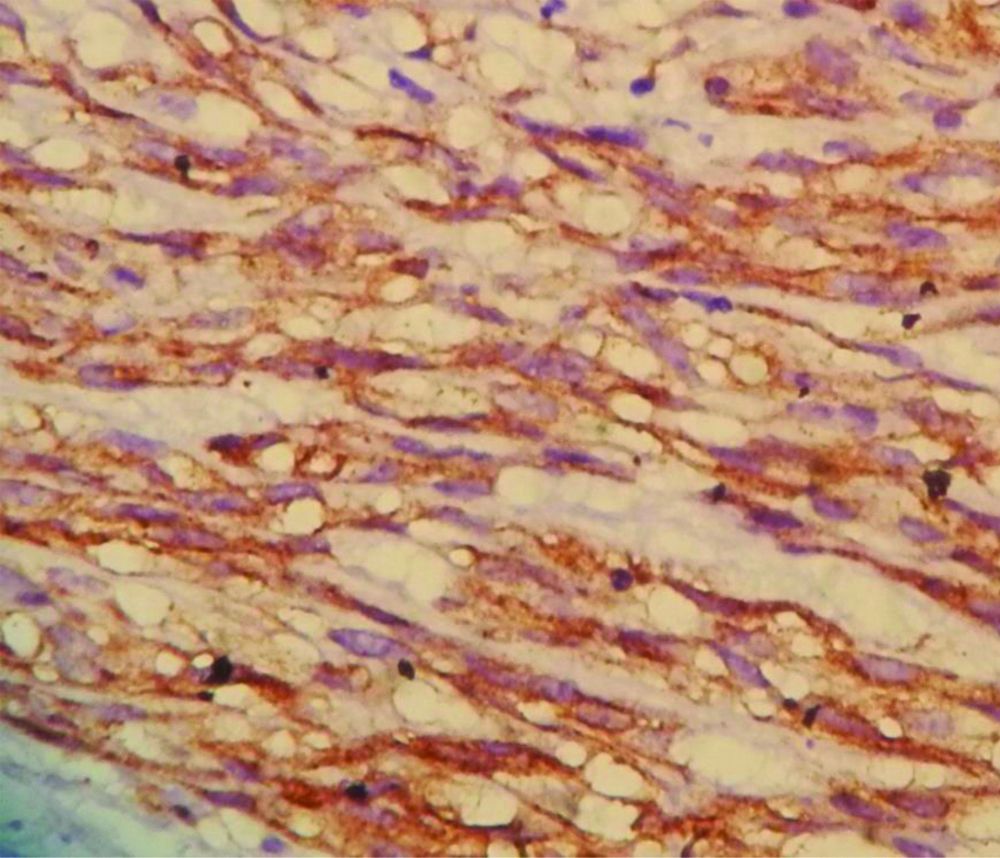
Strong and diffuse cytoplasmic CD117 staining in Malignant GIST (400X).
CD-117: Cancer specific surface antigen; GIST: GastroIntestinal stromal tumors
Discussion
Whipple’s PD procedure is one of the most complex surgeries performed for the management of tumours involving the head of pancreas, duodenum, ampulla of vater and common bile duct [6]. It involves high surgical expertise and is associated with high morbidity and mortality [12].
Gross examination and margin identification: A thorough knowledge of the gross anantomy of PD specimen is necessary for meticulous dissection. For every specimen the clinicoradiological findings should be familiarised by the grossing pathologist.
A Whipple’s PD specimen can be one of the following types
Classic Whipples procedure which contains distal stomach and pylorus.
A pylorus preserving operation wherein the duodenum is transected 1-2 cms distal to the pylorus.
Extended Whipple’s involves retroperitoneal and aortocaval lymph node dissection.
Upon receiving the specimen, proper orientation of the specimen and identification of important margins which are the pancreatic neck margin, uncinate margin and the common bile duct margin is important. Adsay NV et al., in his study mention the identification of a ‘trapezoid’ created by superior mesenteric artery/portal vein vascular bed in the posterio-median aspect of the pancreatic head. The vertical edges on the left and right are made up of pancreatic neck margin and the uncinate margin respectively. Common bile duct is located anterolaterally to the uncinate margin [11]. Rarely, a Whipple’s specimen may be inclusive of a gall bladder and the cystic duct in which case the cut margin will be the hepatic duct margin.
Sectioning the PD specimen: Pancreatic ampullary, distal bile duct and ampullary cancers have different prognosis and staging. In very large tumours of this region accurate identification of the origin of the tumour may be difficult even for most experienced pathologists. The reason for this is the proximity of the anatomical structures. Therefore, we emphasise on careful sectioning of the pancreatic head. In a survey done on approach to reporting of PD specimens by Feakins R et al., only 87% histopathologists distinguished between intrapancreatic bile duct carcinoma from pancreatic carcinoma [13]. Microscopically, the splincter of oddi which represents the circular smooth muscle fibres is thicker in the ampullary part of the duct than around the intrapancreatic part of the common bile duct. Also, ampullary mucosa forms prominent papillary folds. These two important landmark features are appreciated microscopically.
There are many methods for dissecting the head of pancreas [11,14,15]. Here the authors, followed the axial method of dissecting the head of pancreas. The reason for this being, origin and extent of the tumour can be easily identified. Few of the European pathologists follow serial slicing of the pancreatic head perpendicular to the long axis of the duodenum. This method avoids the opening up of the biliary and pancreatic ducts. They recommend this method for its simplicity and retained relationship to the anatomical structures as well as the surgical margins [15-17]. This method of dissection is yet to be implemented.
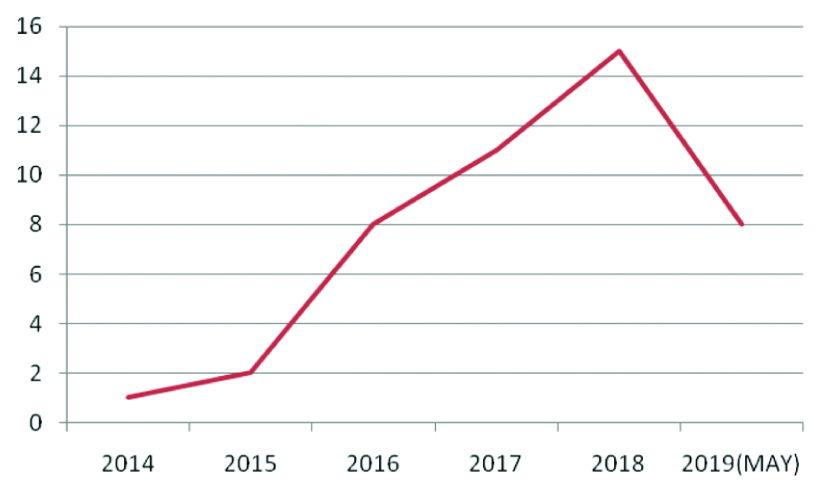
Demography and clinicopathological analysis: In our Institution, there has been a progressive increase in the specimens with 57.8% of cases reported in 2017 and 2018 put together [Table/Fig-13]. The increase can be attributed to an increase in cancer detection of this anatomic region. There was a male preponderance (28 cases-62.2%) in the present study, in concordence with study done by Dhakhwa R and Kafle N [18], Jakhmola CK and Kumar A [19], Saraee A et al., [20]. In a 7-year study done by Saraee A et al., the mean age of patients undergoing PDs was 58.4 years which is in concordance to the mean age of 56.2 in the present study [20]. He J et al., in a study of 2564 PDs showed an increase in the median age from 64 years in 1980s to 68 years in 1990s and 2000s [21]. The commonest age group in the present study study was between 5th and 6th decade. However, the most common age bracket was 6-7th decade in a study done by Jakhmola CK and Kumar A [19]. Jaundice was the most common symptom in this study which is in concordance to most other studies [19,20]. Median size of tumours of periampullary, ampullary and pancreatic tumours were 2 cm, 1.1 cm and 3 cm, respectively. Yeo CJ et al., in their study of 242 periampullary adenocarcinomas found the tumour diameter to be smallest for ampullary and bile duct cancers [22].
Five year trend of Whipple’s PD in our hospital.
Pancreatic, ampullary/periampullary and bile duct carcinomas have different staging criteria as well as varying prognosis. Also, neoadjuvant treatment is recommended for pancreatic cancers [23]. Therefore, it is important to meticulously dissect the specimen in order to accurately categorise the tumour origin. In this study, we employed a strict criteria proposed by Albores-Saavedra J et al., for categorisation of the tumour as ampullary or periampullary [10]. When the epicenter of the tumour is in the ampulla and with a pre-invasive lesion, the tumour was diagnosed as ampullary tumour. Periampullary tumours were diagnosed when the tumour grew in a circumferential manner around the ampulla. Few of the tumours which involved the ampulla as well the perimpullary region were diagnosed as mixed due to our diagnositic inability to categorise it to one of the types. Periampullary carcinoma was the most common tumour followed by pancreatic cancers in concordence with study done by Dhakhwa R and Kafle N [18] and Jakhmola CK and Kumar A [19]. However, in a study done on Western population by Saraee A et al., Yeo CJ et al., Badger SA et al., and Chandrasegaram MD et al., revealed pancreatic tumours to be most common [20,22,24,25]. Categorisation of periampullary/ampullary carcinomas can be intestinal, pancreatico-biliary, mixed or of undifferentiated types is very important. Westgaard A et al., in their study found that pancreatico-biliary histologic type of differentiation was independently associated with a poor prognosis [26]. In the present study, there were only 2 cases of pancreatico-biliary type (4.7%), the remaining being that of intestinal type, similar findings were reported by Dhakhwa R and Kafle N [18]. In the present study, well and moderately differentiated tumours were more common in concordance the study done by Dhakhwa R and Kafle N [18]. Yeo CJ et al., found 88% of the periampullary adenocarcinomas to be of moderate or poor differentiation [22].
Lymph nodes: Lymph node metastasis is an independent adverse prognostic factor in ampullary and periampullary adenocarcinomas [27,28]. In any PD specimen a minimum of 12 lymph nodes should be harvested [7]. The average number of lymph nodes examined was 13. In our institution, we started reporting cancers specimens according to CAP guidelines (college of American pathologists) after 2014. As a result of which few of the specimen had less than minimum lymph node harvest. Rowsell CH et al., improved their lymph node retrieval by radially sectioning the uncinate margin and submitting it entirely for processing [29]. Adsay NV et al., in their study advocate the orange-peel approach of lymph node harvest which increased the mean lymph node number from 6.1 to 14 [30]. In 41.9% (18 cases) lymph nodes were positive for tumour which is similar to the findings reported by Goret CC et al., (43.9%) [31]. In the present study, maximum lymph node positivity was seen in pancreatic tumours (7/13). Yeo CJ et al., in his study reported 61% pancreatic adenocarcinomas of which 72% had nodal involvement [22]. Several study series have reported similar findings of higher incidence of lymph node involvement in pancreatic cancers in comparision to cancers of ampullary and periampullary region [21,23,25].
Margin status: There are many confusing nomenclature in respect to the margins in Whipple’s specimen. In our institution we sample common bile duct, pancreatic neck, uncinate, superior mesenteric vein/portal vein, gastric, duodenal margins, respectively. Apart from these margins, anterior and posterior free surfaces were also sampled before sectioning the specimen.
Different studies have revealed varying reports in the microscopic margin status. The reason for this discrepency is lack of consensus on margins and lack of a uniform standardised protocol. European pathologists follow the “1 mm rule” wherein the tumour at a distance of 1 mm from the inked margin is taken as positive [16,32,33,34]. However, many American pathologists follow 0 mm clearance wherein tumour at the inked margin is considered as R1 [9]. We in our institution follow 0 mm clearance. Margin clearance was achieved in 83.7% cases. Yeo CJ et al., reported tumour free margins in pancreatic and ampullary carcinoma in 71% and 97%, respectively [22]. A significant increase in margin clearance was seen in a study done by Chandrasegaram MD et al., post 2010. They attribute this difference in margin status to improvement in surgical technique [25]. However, following standardised guidelines for grossing and reporting the PD may also have contributed. R1 status was seen in 4 of the pancreatic tumours of which 3 were adenocarcinoma and 1 was a neuroendocrine tumour. R1 status is more commonly seen in pancreatic tumours due to highly infiltrative and dispersed growth pattern of these tumours [35]. In a study done by Badger SA et al., 54.5% cases of head of pancreas had R1 status [24].
Perineural, vascular involvement and staging: Perineural infiltration and lymphovascular invasion are independent prognostic factors for long term survival [36,37]. Dhakhwa R and Kafle N reported a 100% perineural and lymphovascular involvement in their study [18]. Perineural and lymphovascular involvement was seen in 73.3% and 46.7%, respectively in pancreatic cancers. This is in concordence to the study done by Winter JM et al., who reported 91% and 53%, respectively [37]. In the present study, most of the cases were in T2 stage in contrast to Goret CC et al., and Foroughi F et al., and who found most of the cases to be in advanced T3 stage [31,38].
Whipples in non-neoplastic conditions: PD may also be employed in patients with chronic pancreatitis when pain cannot be controlled by other means and when the disease is maximal in the head of the pancreas [1]. In the present study, there were 2 cases (4.4%) where PD was done on clinical suspicion of malignancy. Preoperative work-up in both the patients with clinical history of jaundice did not yield positive biopsy results for malignancy. In both these cases histopathology revealed chronic pancreatitis. Foroughi F et al., reported 7 cases (13.7%) 8 which were negative for malignancy [38]. Barone JE reviewed five papers on PDs and determined a collected incidence of benign diagnosis as 13.1% [39]. Crothers JW et al., reported mimickers of malignancy-autoimmune pancreatitis, paraduodenal pancreatitis and amyloidosis in their case series for Whipple’s resection in non-oncological cases [40]. Due to the anatomic complexity of this region, imaging techniques like Endoscopic Ultrasonography (EUS) and Fine Needle Aspiration (FNA) may fail to give definitive diagnosis [41].
Limitation(s)
Small sample size due to rarity of the procedure, as a result of which bile duct carcinomas were not seen in result of the present study, was the limitation. Long-term follow to study survival trends could not be undertaken due to loss of patients to follow-up.
Conclusion(s)
Whipple’s PD specimens require a thorough knowledge of the anatomy of the region as well as a meticulous grossing of the specimens. Thorough sectioning, careful evaluation of the margins, adequate lymph node sampling and categorisation of the histological types is important in predicting the patient survival outcome. Distinction between the three cancers may be a prerequisite for identification of differences in epidemiology, aetiology and molecular biology. Also, even in the era of advanced imaging modalities, one has to keep in mind the benign mimickers of malignancy which may lead to Whipple’s resection.