Pirfenidone has been approved for the treatment of idiopathic pulmonary fibrosis due to its anti-fibrotic activity. It has been shown to have anti-inflammatory and antioxidant properties apart from being an anti-fibrotic agent. Cytokine storm, severe inflammation and oxidative stress leading to acute respiratory distress syndrome and multiorgan failure are factors causing mortality in patients of COVID-19. This article reports two cases of 35 years and 60-year-old male patients of COVID-19, those were diagnosed by real time Polymerase Chain Reaction (PCR) using nasopharyngeal samples. They were started on pirfenidone 400 mg BD, later increased to 600 mg TDS along with empirical antibiotics, dexamethasone, supplemental oxygen and non-invasive ventilator support. Both the patients improved and were discharged. Both the patients were followed by telemedicine after one week, did not require oxygen at rest and were comfortable at rest in contrast to earlier complaints. Thus, the authors conclude that pirfenidone can be a possible cure for COVID-19 patients, larger trials are required to confirm its efficacy.
Antifibrotic, Anti-inflammatory, Coronavirus disease-2019, Respiratory distress
Case Report 1
A 35-year-old male patient presented to Emergency Department with complaints of fever, shortness of breath and dry cough for seven days. Owing to the current pandemic scenario of COVID-19 his nasopharyngeal swab was sent for Real Time Polymerase Chain Reaction (RT-PCR) testing of COVID-19 which came out to be positive.
Patient was shifted to COVID-19 isolation ward of the hospital. His room air saturation (spo2) by pulse oximeter at presentation was 70% on room air following which the patient was given supplemental oxygen, following which his saturation was 80%. In the isolation ward, his routine investigations were done which included Complete Blood Count (CBC), Renal Function Tests (RFT), Liver Function Test (LFT), lipid profile, Arterial Blood Gas sample (ABG), D-dimer, troponin I, C-reactive protein, N-terminal pro B-type Natriuretic Peptide (NT pro BNP), Haemoglobin A1c (HbA1c) and chest x-ray. In ABG arterial oxygen level (pO2) was found to be very low (40 mmHg), his D-dimer value was raised (779 ng/mL) and hbA1c was also raised (7.5). C-reactive Protein (CRP) was also raised. He was not a known case of type 2 diabetes mellitus so it was a newly diagnosed Type 2 Diabetes Mellitus (T2DM). Rest of the reports like CBC, RFT, LFT, lipid profile, Troponin I and NT-pro BNP were within normal range. His chest X-ray showed bilateral consolidation in all zones of both lungs [Table/Fig-1]. Since his D-dimer was raised, respiratory rate was 46/min and Arterial Blood Gas (ABG) showed marked hypoxia so Computed Tomography (CT) pulmonary angiography with High-Resolution CT (HRCT) thorax was planned to rule out pulmonary embolism and to see the extent of involvement of lung parenchyma.
Chest radiograph of patient 1 showing infiltrates involving all zones of both the lungs.
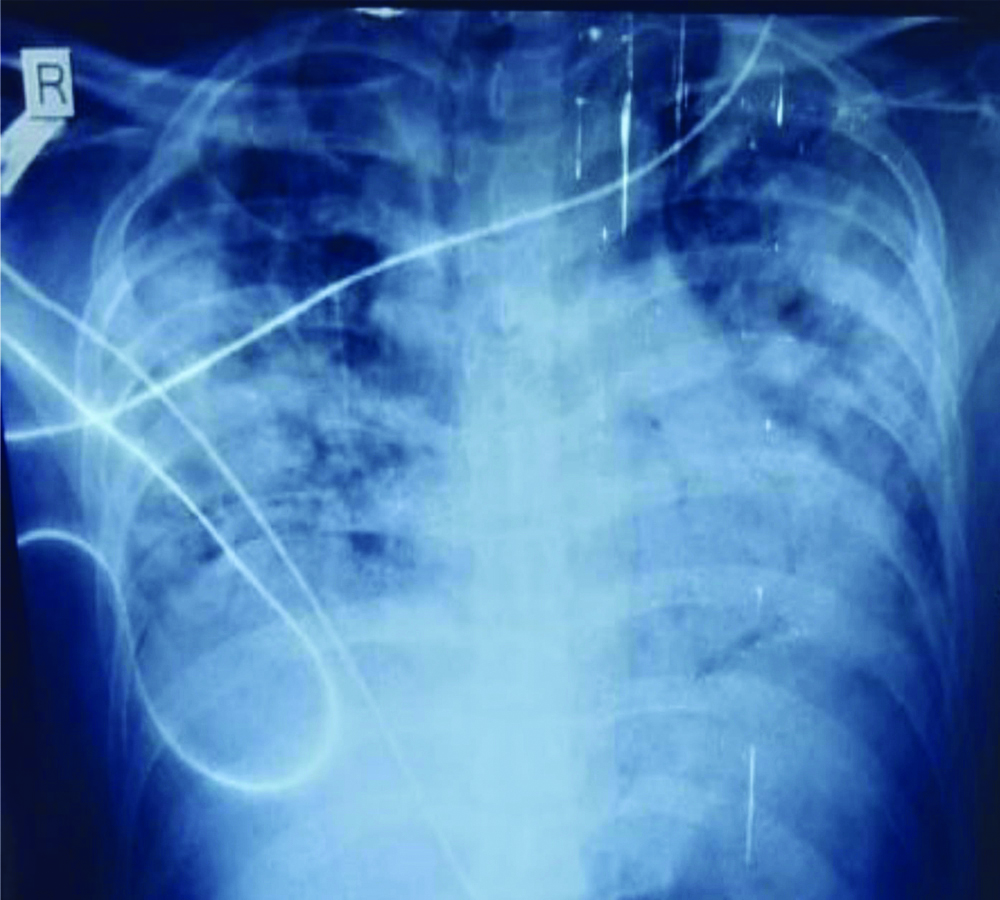
Patient was started on symptomatic treatment for COVID-19. He was given empirical antibiotics meropenem and moxifloxacin alongwith doxycycline 100 mg BD and ivermectin 6 mg BD for five days. Injectable dexamethasone 6 mg BD was also given. Blood culture report was done which showed growth of staphylococcus sensitive to linezolid so it was added to treatment given. Insulin was started according to sliding scale for diabetes mellitus.
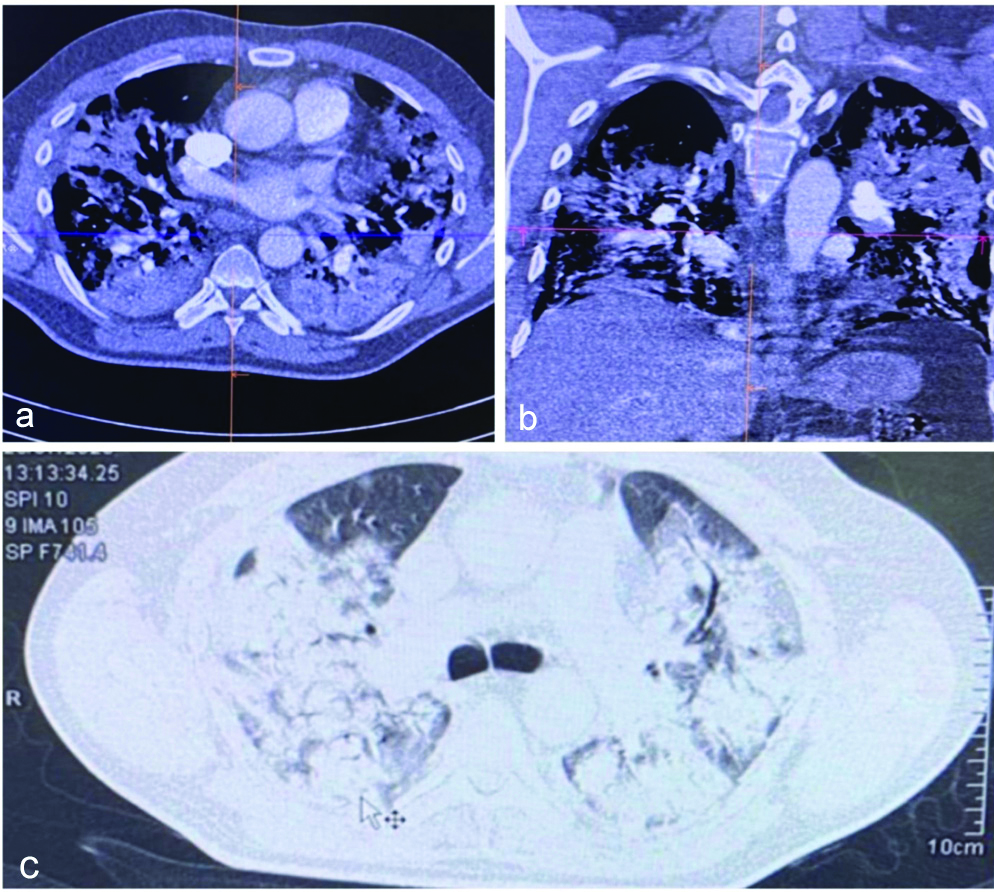
His CT scan showed large confluencing high attenuation soft tissue density patches with multiple linear hypodensity within it in the background of ground glass haziness diffusely involving bilateral lung with predominantly involving bilateral perihilar region and lower lobes [Table/Fig-2]. There was no evidence of pulmonary embolism.
CT scan of patient 1 showing consolidation, soft tissue density patches with ground glass haziness involving both the lungs.
Patient was given non-invasive ventilator support in the Continuous Positive Airway Pressure (CPAP) mode alongwith supplemental oxygen but his saturation did not improve much and his respiratory rate was high (46/min). As the patient was not maintaining saturation, he was given high flow oxygen and his saturation reached the level of 90-92% but only for the duration when he was on high flow oxygen following the removal of which his saturation dropped and his po2 in ABG still remained low 58 mm Hg.
This treatment was continued for about a week but neither the room air saturation by pulse oximeter (spO2) nor po2 of the patient improved to a satisfactory level and his respiratory rate remained high. So, it was planned to start the anti-fibrotic drug pirfenidone 400 mg BD. It was started on lower dose and later on when there were no side-effects and no derangements of LFT the dose was increased to 600 mg TDS. After four days of starting this drug his oxygen requirement started to decrease gradually and his respiratory rate improved, his pO2 also improved in ABG. Patient started mobilising after 10 days of starting the drug was maintaining 94% saturation at 2 L/m of oxygen with nasal cannula and his respiratory rate came down to 20/min and pO2 improved to 78 mm Hg. His RT-PCR for COVID-19 came out to be negative after eight days of admission and he was shifted to respiratory ward ICU where he was later managed and discharged around after around 2-3 weeks of testing on symptomatic improvement.
The patient was not maintaining room air saturation >94% so was discharged with home-based oxygen therapy. Pirfenidone was advised for 10 weeks. CT scan was not repeated at the time of discharge.
Case Report 2
A 68-year-old male patient presented with complains of shortness of breath for 15 days, fever and dry cough for two days. He was known case of type2DM and systemic hypertension for 10 years. His nasopharyngeal sample was positive for COVID-19 by RT-PCR. Patient was shifted to isolation ward and his routine investigations were also sent. His room air saturation (spO2) by pulse oximeter at presentation was 78% on room air and he was given supplemental oxygen following which he maintained a saturation of 92% on 15L/m supplemental oxygen. Patient was a known diabetic and hypertensive for last 10 years and was already on medication for those comordities. His pO2 was also found to be very low (28 mmHg) in ABG, D-dimer was raised >10000 ng/mL, CRP was raised, and his respiratory rate was 42/min, rest of the routine investigations like haemogram, RFT, LFT, and lipid profile were normal. Chest x-ray showed diffuse involvement of all zones of both lungs [Table/Fig-3]. CT pulmonary angiography with HRCT thorax was also done for this patient to rule out pulmonary embolism owing to clinical scenario.
Chest radiograph of patient 2 showing infiltrates involving all zones of both lungs.
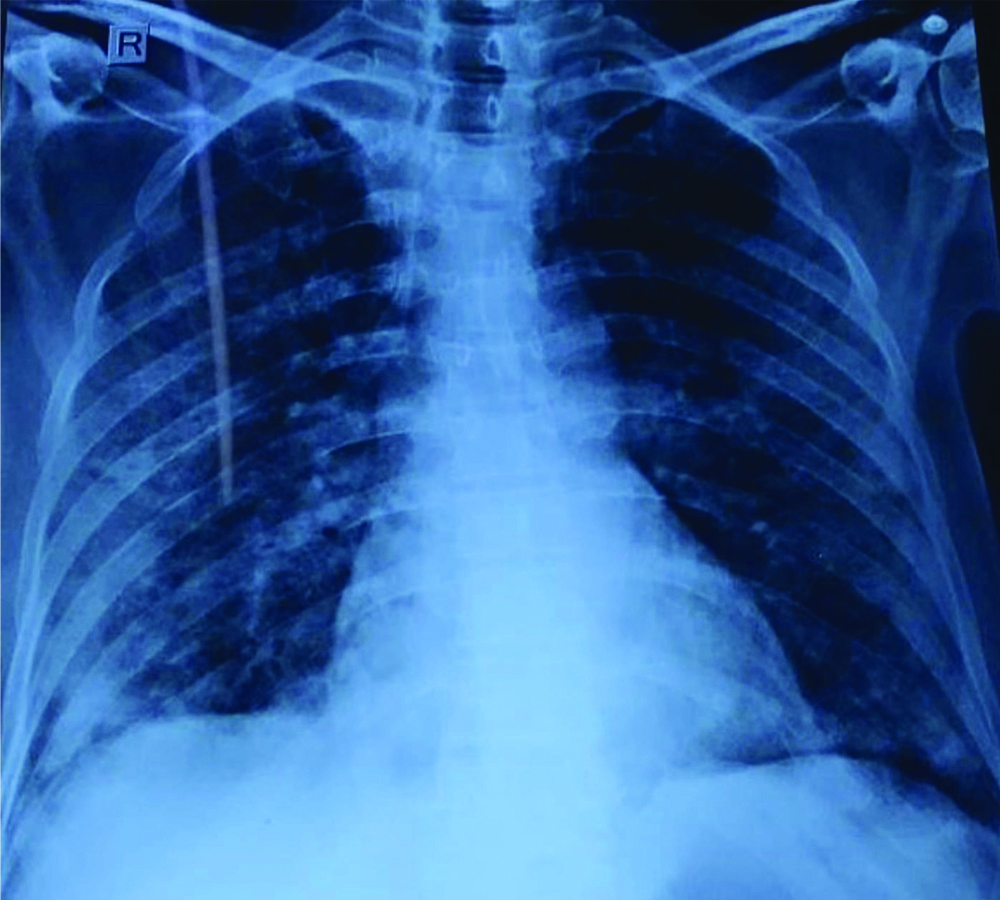
CT scan showed consolidatory patches with adjacent ground glass patches and nodules and interlobular and intralobuar septal thickening diffusely involving bilateral lung [Table/Fig-4]. There was no evidence of pulmonary embolism.
CT scan of patient 2 showing consolidatory patches with ground glass haziness involving both the lungs.
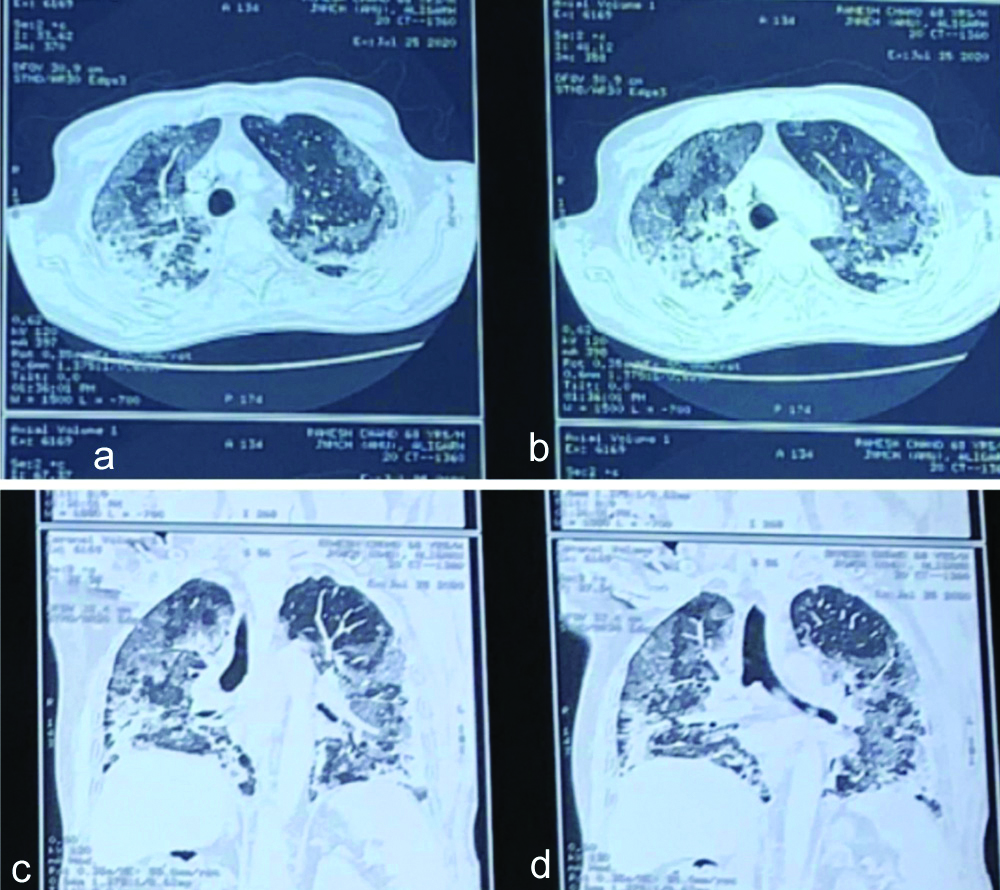
Patient was started on symptomatic treatment for COVID-19 along with injection dexamethasone 6 mg iv BD and supplemental oxygen and non-invasive ventilator support in CPAP mode. This was continued for one week but pO2 of the patient did not improve much and respiratory rate was also high even after continuous CPAP support. So, next the patient was put on tablet pirfenidone 400 mg BD which was later increased to 600 mg TDS after patient tolerated the drug and LFT remained normal. After one week of starting the drug his oxygen requirement decreased to 3L/m, maintained saturation of 96% on 3L/m, his pO2 improved to 82 mmHg and respiratory rate came down to 24/min. We were able to taper down CPAP duration and pressure support given and he was discharged after two weeks after testing negative by RT-PCR on home-based oxygen and pulse oximeter to monitor the spO2 levels. Pirfenidone was advised for 10 weeks and CT scan at discharge was not done.
Interleukin-6 (IL-6) levels were not done in both the patients. Both the patients were followed by telemedicine after one week and told that they did not have any new complaints and required supplementary oxygen only for few hours (6-8 hours) of the day particularly after doing some activity like after coming from washroom or after bathing and during night time. Both the patients did not require oxygen at rest and were comfortable at rest as opposed to the when they were started on treatment (they were dyspneic even at rest).
Discussion
Acute Respiratory Distress Syndrome (ARDS) and multiorgan damage are due to inflammation, oxidative stress, increased permeability of vascular bed and reactive oxygen species damage and cytokine storm in patients of COVID-19 particularly those who are elderly and have other comorbidities, the most common being diabetes mellitus and other such as systemic hypertension, malignancy etc., [1].
In search for the treatment of COVID-19, pirfenidone has been found to have a possible role in treatment because of its anti-fibrotic activity along with anti-inflammatory and antioxidant effects [2-4].
The spectrum of fibrotic changes in COVID-19 patients range from organising pneumonia to severe acute lung injury leading to various widespread fibrotic changes in lungs, hence, it is rationale to use anti-fibrotic drugs in patients of COVID-19 [5]. Pulmonary fibrosis has been seen at autopsy of severe COVID-19 cases [6].
Tumour necrosis factor–alpha (TNF-α) is a well-known mediator of inflammation and pirfenidone has been found to be inhibitor of TNF-α by many authors such as Shi Q et al., and Hale Martha L et al., [7,8]. NLRP3 is a multimeric protein complex that initiates an inflammatory reaction with the release of pro-inflammatory cytokines like IL-1 beta and IL-18. In a study done by Li Y et al., they found that pirfenidone blocks NLRP3 inflammosome (NLR family pyrin domain containing 3) activation thus, prevents lipopolysaccharide induced pulmonary inflammation and fibrosis [9]. Pirfenidone inhibits formation of collagen 1 fibril [10]. The principal action of pirfenidone as antifibrotic agent is primarily due to reduction in overexpression of TGF-β [11]. Damage to lipids and proteins, cell apoptosis, ADP-ribosylation, injury to mitochondrial DNA, and impaired nitric oxide activity, cytoskeletal damage and lipid peroxidation are destructive effects of inflammation and severe oxidative stress due to cytokine storm [3,4]. It has been shown that pirfenidone could inhibit NADPH-dependant lipid peroxidation [12]. The antioxidant effect of pirfenidone is helpful in treatment of hyperimmune response [3,4]. The receptors for COVID-19 are ACE receptors and pirfenidone decreases angiotensin converting enzyme, angiotensin II and expression of type I angiotensin II receptors, it also inhibits AT1R/p38 MAPK pathway and increases expression of liver X receptor-α expression [13], thus decreasing the chance of COVID-19 virus entry into the cells as well as preventing development of fibrosis. Xi Z et al., also reported improvement in saturation and dyspnea of the patient with administration of pirfenidone [14].
The rationale for using corticosteroids in severe and critical COVID-19 patients is that early and judicious use of steroids help in improving the ventilator parameters, prevent progression to ARDS and death [15], help in reducing incidence of intubation, help in quick defervescence, improve oxygenation [16] and ventilation and hence decrease the incidence of intubation [17]. Overall, it helps in reducing mean duration of hospital stay.
Conclusion(s)
Till date there has been no proven drug or vaccine for treatment of COVID-19. From the knowledge of pathophysiology of COVID-19 and knowledge of mechanisms of action of pirfenidone, drug should be used only in case-to-case basis after giving all the information to the patient and relatives. Also, it is better to have more information before routine use of this drug can be recommended. Hence, need larger experimental trials are required to document the efficacy of pirfenidone in patients with moderate-to-severe COVID-19.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 09, 2020
Manual Googling: Oct 07, 2020
iThenticate Software: Dec 14, 2020 (17%)
[1]. Wu D, Yang XO, TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor FedratinibJournal of microbiology, immunology, and infection=Wei mianyugan ran za zhi 2020 10.1016/j.jmii.2020.03.00532205092 [Google Scholar] [CrossRef] [PubMed]
[2]. Soroush S, Amirhossein K, Shervin T, Shima A, Hamidreza A, Alireza G, Effect of pirfenidone on pulmonary fibrosis due to paraquat poisoning in ratsClin Toxicol 2012 50(8):754-58.10.3109/15563650.2012.71878322924653 [Google Scholar] [CrossRef] [PubMed]
[3]. Fois AG, Posadino AM, Giordo R, Cossu A, Agouni A, Rizk NM, Antioxidant activity mediates pirfenidone antifibrotic effects in human pulmonary vascular smooth muscle cells exposed to sera of idiopathic pulmonary fibrosis patientsOxidative Medicine and Cellular Longevity 2018 2018:263908110.1155/2018/263908130420906 [Google Scholar] [CrossRef] [PubMed]
[4]. Giri SN, Leonard S, Shi X, Margolin SB, Vallyathan V, Effects of pirfenidone on the generation of reactive oxygen species in vitroJ Environ Pathol Toxicol Oncology 1999 18(3):169-77. [Google Scholar]
[5]. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive studyLancet Infect Dis 2020 20(4):425-34.10.1016/S1473-3099(20)30086-4 [Google Scholar] [CrossRef]
[6]. Zhang T, Sun LX, Feng RE, Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019ZhonghuaJie He He Hu Xi Za Zhi 2020 43(6):e040 [Google Scholar]
[7]. Shi Q, Liu X, Bai Y, Cui C, Li J, Li Y, In vitro effects of pirfenidone on cardiac fibroblasts: Proliferation, myofibroblast differentiation, migration and cytokine secretionPloS one 2011 6(11)10.1371/journal.pone.002813422132230 [Google Scholar] [CrossRef] [PubMed]
[8]. Hale Martha L, Margolin Solomon B, Teresa K, Roy Chad J, Stiles Bradley G, Pirfenidone blocks the in vitro and in vivo effects of staphylococcal enterotoxin BIAI 2002 70(6):2989-94.10.1128/IAI.70.6.2989-2994.200212010989 [Google Scholar] [CrossRef] [PubMed]
[9]. Yi L, Haitao L, Shuai L, Pinhua P, Xiaoli S, Hongyi T, Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activationMol Immunol 2018 99:134-44.doi: 10.1016/j.molimm.2018.05.003 [Google Scholar]
[10]. Larissa K, Yoshihiro I, Michaela A, Katharina H, Rudolf H, Jürgen B, A novel antifibrotic mechanism of nintedanib and pirfenidone. inhibition of collagen fibril assemblyAm J Respir Cell Mol Biol 2017 57(1):77-90.10.1165/rcmb.2016-0217OC28257580 [Google Scholar] [CrossRef] [PubMed]
[11]. Yang Y, Ye Y, Lin X, Wu K, Yu M, Inhibition of pirfenidone on TGF-beta2 induced proliferation, migration and epithlial-mesenchymal transition of human lens epithelial cells line SRA01/04PloS one 2013 8(2):e5683710.1371/journal.pone.005683723437252 [Google Scholar] [CrossRef] [PubMed]
[12]. Misra HP, Rabideau C, Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicalsMol Cell Biochem 2000 204(1-2):119-26. [Google Scholar]
[13]. Li C, Han R, Kang L, Wang J, Gao Y, Li Y, Pirfenidone controls the feedback loop of the AT1R/p38 MAPK/renin-angiotensin system axis by regulating liver X receptor-alpha in myocardial infarction-induced cardiac fibrosisSci Rep 2017 7:40523https://doi.org/10.1038/srep4052310.1038/srep4052328091615 [Google Scholar] [CrossRef] [PubMed]
[14]. Xi Z, Zhigang Z, Ting L, Post inflammatory pulmonary fibrosis in a discharged covid 19 patient: Effectively treated with pirfenidoneArch Pulmonol Respir Care 2020 6(1):51-53. [Google Scholar]
[15]. Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, Early short course corticosteroids in hospitalized patients with COVID-19medRxiv preprintdoi: 10.1101/2020.05.04.20074609(not certified by peer review) [Google Scholar]
[16]. Wang Y, Jiang W, He Q, Liu B, Dong N, Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, ChinamedRxiv preprintdoi: 10.1101/2020.03.06.2003234210.1101/2020.03.06.20032342 [Google Scholar] [CrossRef]
[17]. Chorobozcek T, Lacoste M, Wackenheim C, Challan Belval T, Amar B, Boisson T, Beneficial effect of corticosteroids in severe COVID-19 pneumonia: A propensity score matching analysismedRxiv preprint[not certified by peer review10.1101/2020.05.08.20094755 [Google Scholar] [CrossRef]