It has been documented that there is a release of melanin pigment from Iris Pigment Epithelium (IPE) in PDS patients’ eyes, which is deposited on Trabecular Meshwork (TM), lens zonules, lens surface, iris surface and corneal endothelium by aqueous convection currents. Accumulation of pigments in TM is responsible for increased aqueous outflow resistance, which may lead to PG [2,3].
Studies done earlier showed the varied clinical features and prevalence of PDS, in different racial group. Among adult whites, the prevalence of PDS was 2.45% [1,4-7]. In a study [9] among black population (>7 years of age), the prevalence of PDS was found to be 0.167%±0.013. The black and white PDS patients have different spectrum of clinical signs like ITDs and anterior iris stromal pigment dusting were not found common in black patients as in whites [8-13].
The Caucasians of age between 20 to 45 years are more commonly affected by PDS. It is relatively less common in Asians and Africans. PDS patients with sustained IOPs >21 mmHg and no optic nerve changes or visual field loss should probably be treated with IOP-lowering therapy [14]. The aim of this study was to evaluate clinical characteristics of PDS and PG patients in eastern part of Uttar Pradesh.
Materials and Methods
This was a hospital based retrospective cross-sectional study done at a tertiary eye care center of Regional Institute of Ophthalmology, Sitapur, Eastern Uttar Pradesh region. The study was approved by the Institutional Review Board of the parent institution and adhered to the tenets of the Declaration of Helsinki, (IEC No- SHE/2019.20/640). Informed consent was obtained from all patients before undertaking treatment options.
The records of all patients who were diagnosed, either as PDS or PG, from 1st January 2018 to 31st December 2019 (2 years), were analysed and included in this study.
Inclusion and Exclusion criteria: Patients who underwent a complete ophthalmic examination, with complete records and clinical finding consistent with either PDS or PG or both, were included in this study. Patients with any previous ocular surgery, laser treatment, uveitis, any ocular trauma, exfoliative material and pseudo exfoliation were excluded from the study.
The patients were divided into PDS and PG group. Diagnosis of PDS was made when at least two of the following three signs were found clinically: Krukenberg spindle, homogenous moderate-to-heavy (≥Spaeth 2+) TM pigmentation, and any degree of zonular, and/or lenticular pigment granule dusting, with normal Optic Nerve Head (ONH) and normal visual field and with or without increased IOP. PDS may or may not be associated with increased IOP [15]. Patients with PDS were diagnosed with PG, if they had two or more of the following findings: initial IOP >21 mmHg, glaucomatous optic nerve damage or glaucomatous visual field loss.
Comprehensive ophthalmic examinations included visual acuity measurement by Snellen’s chart and converted to corresponding log Minimum Angle of Resolution (MAR) values, refraction, IOP measurement by Goldmann applanation tonometer, slit-lamp biomicroscopy pre- and postmydriasis, gonioscopy, fundus examination, peripheral retinal examination and automated Humphrey Swedish Interactive Threshold Algorithm (SITA) standard 24-2 visual field test, or by Humphrey automated 10-2 SITA standard perimetry if their glaucomatous damage was very severe. Systemic and ocular medical history of each subject was also recorded.
All the patient were evaluated for the corneal endothelial pigmentation, anterior iris stromal pigment dusting, ITDs, posterior iris bowing (concave iris configuration), increased TM pigmentation, pigment granule dusting on lens zonules or peripheral posterior surface of lens, peripheral retinal degeneration and pigmentation and optic disc examination. Pigmentation of TM was leveled as 0 no pigment, 1+ minimal, 2+ mild, 3+ moderate and 4+ intense, by Spaeth grading system [16]. Pigment deposition on corneal endothelium was depicted as Krukenberg spindle, diffuse pigmentation or no pigmentation. Each patient’s Central Corneal Thickness (CCT) was measured using an ultrasonic pachymeter. The average of five CCT values was taken.
Sample Size
Sample size was calculated by using formula, given below:
n=z2×p×(1-p)/e2
Where, z=1.96 for a confidence level (α) of 95%, p=proportion, e=margin of error, n=sample size;
z=1.96, p=0.02 (2%), e=0.05 (5%)
n=1.962×0.02×1-0.02)/0.052
n=0.0753/0.0025=30.118
n=0.0753/0.0025=30.118, n≈31
The sample size came out to be approximately 31.
Statistical Analysis
All statistical analyses were done at 5% significance using Graph Pad Instat version 3.0 and Microsoft Excel 2019. Descriptive analysis such as mean, standard deviation and percentage were used to exhibit the clinical parameters. A p-value less than 0.05 considered statistically significant.
Results
Out of 2012 patients, examined in glaucoma specialty clinic during the 2-years period of time, a total of 40 patients were diagnosed as having either PDS or PG. Out of total 40 patients, 16 patients were diagnosed as PDS and 24 as PG. Out of total 80 eyes, 39 eyes had PDS and 41 eyes had PG. Thirty one (77.5%) were male and nine (22.5%) patients were female. Mean age of study patients was 36.52±8.91 years (range, 16-57) with male to female ratio 3.4:1. The average age of PDS and PG patients were 36.5±11.76 and 36.5±6.34 years, respectively. There was no statistically significant difference between age of PDS and PG patients (p=1.0). Demographic characteristics are summarised in [Table/Fig-1].
General characteristics of study patients.
| Variable | All study patients | PDS | PG | p-value (PDS vs PG) |
|---|
| Total No. of patients | 40 | 16 | 24 | - |
| Total No. of eyes | 80 | 39 | 41 | - |
| Male/Female | 31/9 | 11/5 | 20/4 | 0.44* |
| Male:Female ratio | 3.4:1 | 2.2:1 | 5:1 | - |
| Age (years) | 36.52±8.91 | 36.5±11.76 | 36.5±6.34 | 1.0$ |
| Male | 36.1±8.7 | 34.9±12.1 | 36.8±5.63 | 0.5534$ |
| Female | 37.9±10.5 | 40±10.13 | 35.25±8.98 | 0.4853$ |
PDS: Pigment dispersion syndrome; PG: Pigmentary glaucoma; *Chi-square test
$unpaired t test, statistical significance level ≤0.05
Average Best Corrected Visual Acuity (BCVA) (log MAR) of all the patients was 0.36±0.75 while among PDS and PG patients, it was 0.038±0.15 and 0.58±0.89, respectively (p=0.0001). The mean CCT (μm) of all the study patients was 538.6±27.82 while for PDS and PG patients, it was 550.18±23.10 and 530.87±28.03, respectively (p=0.0280) [Table/Fig-2]. All patients except nine eyes of the six patients had myopia of -0.5D or greater, with mean refractive error of -3.55±4.72 spherical equivalent (range, -24.75 to 0.5). The mean refractive error of PDS patients was -1.22±1.49 (range, -4.5 to +0.5D) and in PG eyes was -5.10±5.44 spherical equivalent (range, -24.75 to -0.25D), (p=0.0085), [Table/Fig-2]. The average baseline IOP in all study patients was 30.21±11.42 mmHg (range 12-56). The average IOPs in PDS and PG patients were 21.75±8.27 mmHg and 35.85±9.61 mmHg, respectively (p=0.001) [Table/Fig-2]. Thirty six out of forty patients had increased initial IOP of >21 mmHg in at least one eye at the time of diagnosis and 62 eyes out of 80 eyes had initial IOP of >21 mmHg. The average baseline MD and average VFI (%) of all study patients, on Humphrey visual field analysis were -7.71±8.29 and 83.58±22.24, respectively. In PDS and PG group average baseline MD were -0.423±1.18 and -12.9±7.21, respectively (p=0.0001). The VFI (%) were 98.03±1.62 and 73.95±24.31 in PDS and PG groups, respectively (p=0.0003) [Table/Fig-2].
Baseline Investigation findings in study patients.
| Variables (Mean±SD) | All study patients (n=40) | PDS (n=16) | PG (n=24) | p-value (PDS vs PG) |
|---|
| BCVA (log MAR) | 0.36±0.75 | 0.038±0.15 | 0.58±0.89 | 0.0001$ |
| CCT (μm) | 538.6±27.82 | 550.18±23.10 | 530.87±28.03 | 0.0280$ |
| Baseline IOP (mmHg) | 30.21±11.42 | 21.75±8.27 | 35.85±9.61 | 0.0001$ |
| C:D ratio | 0.667±0.235 | 0.478±0.134 | 0.793±0.202 | <0.001$ |
| Baseline MD | -7.71±8.29 | -0.423±1.18 | -12.9±7.21 | 0.0001$ |
| VFI (%) | 83.58±22.24 | 98.03±1.62 | 73.95±24.31 | 0.0003$ |
| Spherical equivalent | -3.55±4.72 | -1.22±1.49 | -5.10±5.44 | 0.0085$ |
BCVA: Best corrected visual acuity; CCT: Central corneal thickness; MD: Mean deviation; VFI: Visual field index; $unpaired t test, statistical significance level ≤0.05
Five patients had family history of glaucoma and four had family history of PDS. Ten patients had been diagnosed with ‘primary open angle glaucoma’ before referral to the hospital. All of them were taking anti-glaucoma medications at the time of evaluation for PDS. Only nine of the PDS patients had symptoms, which were mostly occasional blurred vision accompanied with heaviness of the affected eye and headache.
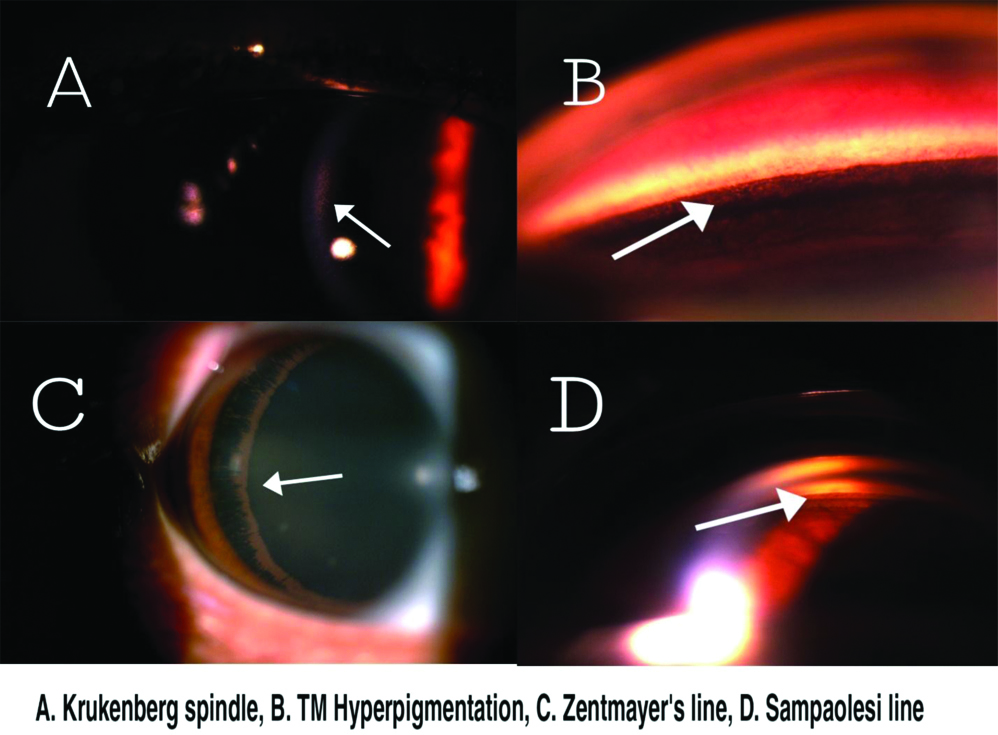
Out of 40 patients, thirty three patients (82.5%) had Krukenberg spindles in at least one of the eye, which were bilateral in 28 patients and unilateral in five patients. Six (one PDS and five PG) patients had traces of diffuse corneal endothelial pigmentation. One patient of PDS did not have any corneal endothelial pigmentation in both eyes [Table/Fig-3].
Clinical findings of anterior segment in study patients.
| Variables | PDS | PG |
|---|
| Corneal endothelial pigmentation |
| KS | 14 (35%) | 19 (47.5%) |
| DP | 1 (2.5%) | 5 (12.5%) |
| None | 1 (2.5%) | - |
| TM pigmentation |
| 0 no pigment | - | - |
| 1+ minimal | - | - |
| 2+ mild | 1 (2.5%) | - |
| 3+moderate | 9 (22.5%) | 10(25%) |
| 4+ intense | 6 (15%) | 14 (35%) |
| ITDs |
| Traces | - | 1 (2.5%) |
| Lenticular/zonular pig |
| Traces | 5 (12.5%) | 2 (5%) |
| Grd 1 | 9 (22.5%) | 5 (12.5%) |
| Grd 2 | 1 (2.5%) | 6 (15%) |
| Grd 3 | 1 (2.5%) | 10 (25%) |
| Grd 4 | - | 1 (2.5%) |
| Anteriror Iris stromal pigment dusting |
| Traces | - | 2 (5%) |
| Mid-peripheral iris configuration |
| Concave | 2 (5%) | 10(25%) |
| Flat | 14 (35%) | 14 (35%) |
KS: Krukenberg spindle; DP: Diffuse pigmentation; TM: Trabecular meshwork; ITD: Iris trans-illumination defect; Grd: Grade
Twelve (2 PDS and 10 PG) patients had concave mid-peripheral iris configuration and 28 (14 PDS and 14 PG) patients had flat mid-peripheral iris configuration. Only two patients (PG patients) had a trace cluster of pigment granules on inferior iris surface and no patient exhibited an obvious diffuse anterior iris stromal pigment granule dusting. Typical spoke-like radial ITDs were not observed in any of the patients [Table/Fig-3]. In one patient, the most myopic of the group with -24.75D spherical equivalent had, isolated short slit-like trans-illumination defects in iris crypts.
Homogeneous TM pigmentation was seen in all patients and Sampolesi’s line was visualised at almost 360 degrees in all cases on gonioscopy [Table/Fig-4]. All study patients showed Zentmayer line (also known as Scheie’s line). Zentmayer line represents pigment granule deposition on lens zonules or on posterior peripheral surface, which may be of different extent [Table/Fig-4].
Clinical pictures of anterior segment findings.
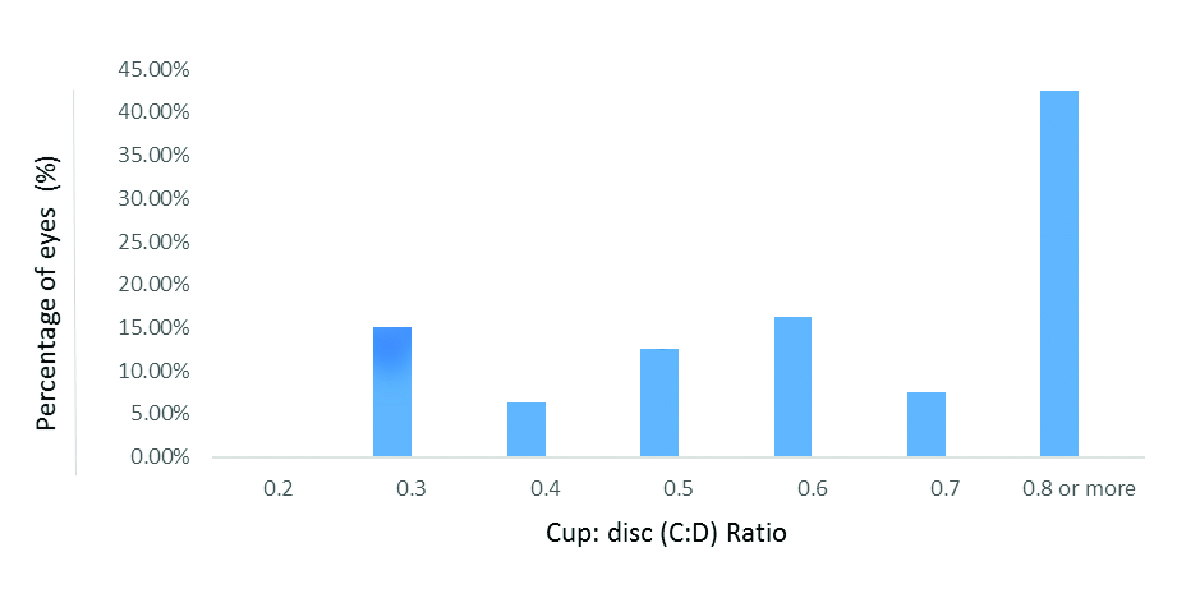
On fundus examination 34 (42.5%) eyes had C:D ratio of 0.8 or more, 6 (7.5%) had C:D ratio of 0.7 and 40 (50%) eyes were having C:D ratio of 0.6 or less, as shown in [Table/Fig-5]. The average C:D ratio in all study patients was 0.667±0.235. The C:D ratio of PDS and PG patients, were 0.478±0.134 and 0.793±0.202, respectively, (p=<0.001) [Table/Fig-2].
Cup:disc (C:D) ratio distribution among eighty eyes of the study population.
Notably, 60% (24/40) patients, either in one or both eyes had glaucoma manifestations of secondary to PDS at the initial diagnosis. Seventeen of them were diagnosed as having PG in both eyes and seven patients were diagnosed with PG, unilaterally. Three patients had BCVA of Light Perception (LP) in one eye and two patients had no LP in one eye. Both eyes of one patient were having <20 degrees of visual field on Humphrey perimetry, at initial examination.
Discussion
The objective of the present study was to evaluate clinical characteristics of PDS and PG patients at a tertiary eye care center in Eastern Uttar Pradesh and it was found that, 82.5% of the study patients had Krukenberg spindles. In black patients, this percentage was 57.1% [8]. In our study, TM pigmentation was homogeneous and pigment granule deposition on posterior surface of lens and zonules were most common findings and these similar findings were also common among black population [8], while Spoke-like mid-peripheral ITDs were rarely found in the present study and Mid-peripheral iris concave configuration was common among 30% of the study patients. In a study done by Roberts DK et al., the black patients did not show obvious concave iris configuration rather showed flat peripheral iris configuration and deep anterior chamber depth. Trace cluster of pigment granules on inferior iris surface were identified in two of the forty (5%) study patients, but was not reported in black patients [8]. The white patients also have concave iris configuration, pigment deposition on zonules and/or on posterior lens surface [4,6,7,17]. The black patients are different from white patients in not having mid-peripheral radial ITDs [8-13].
Typical spoke-like radial ITDs were not detected in any of the study patient. Whilst the most myopic of the group with -24.75 D spherical equivalent, isolated short slit-like trans-illumination defects in iris crypts was observed in one (2.5%) patient.
The anterior iris stromal pigment granule dusting is not as common in black patients as compared to white patients. Trace cluster of pigment granules on inferior iris surface were identified in two out of the forty (5%) study patients, but was not reported in black patients.
The mean age of the study population was found to be 36.52±8.91 years with 3.4:1 male to female ratio. No statistically significant difference in age (p=1.0) between PDS and PG patients was found. In both the groups, males were affected more than females. Qing G et al., reported that, mean age of the PDS patients was 35.5±7.0 years (range, 22-49), with a 2:1 male-to-female ratio [18]. Age was significantly (p<0.0001) lower and there were significantly (p<0.001) more men than women in the group with PG compared with the group with primary open angle glaucoma [19].
The lack of ITDs in black PDS patients may be due to their dark irides [8,11,13]. The iris color of Indians is usually brown or dark brown, which results from heavy pigmentation in iris melanocytes and iris stroma by virtue of which they can block an IPE trans-illumination defect. The heavily pigmented irides in PDS/PG patients may also account for the lack of ITDs in PDS.
In the best of the author’s knowledge, there has been no separate study conducted regarding clinical features of PDS/PG in patients of eastern Uttar Pradesh. In this study, it was found that study patients comprised of 1.98% (40 of 2012) of all outpatients in glaucoma specialty clinic of the hospital. Similar results were reported by Qing G et al., where PDS patients comprised 1.1% (18 of 1632) of all outpatients in glaucoma specialty clinic of Beijing [18]. This turned out to be much more common than we had imagined. The lack of typical ITDs in Indian PDS/PG patients may distract the attention of clinicians from suspecting PDS.
Ten study patients were diagnosed with primary open angle glaucoma before referral to the glaucoma clinic. Although, 82.5% (33 out of 40) were detected having Krukenberg spindle. It is subtle and hard to notice in some patients even under slit-lamp bio-microscopy because of the dark background of the highly pigmented iris, especially when the pigmentation remains minimal. The anterior iris stromal pigment granule dusting was absent in the majority of the study patients.
In the study, 60% (24/40) patients, had glaucoma manifestations of secondary to PDS at the initial diagnosis and 90% patients had raised initial IOP. Seventeen patients of them were diagnosed as having PG in both eyes and seven were diagnosed with PG unilaterally. Many PDS patients are not detected until they develop PG or visual symptoms due to incomplete and subtle spectrum of clinical signs in them. These percentages are much higher than white PDS patients [4,6,7,17,20].
In the present study, the mean refractive error of PDS and PG patients was -1.22±1.49 and -5.10±5.44 spherical equivalent, which was significantly more myopic in PG patients, (p<0.0085). Gramer E et al., reported that greater the myopia, the earlier the onset of glaucoma [21]. Another study by Jonas JB et al., showed that refractive error was significantly more myopic (p<0.0001) in the group with PG compared with the group with primary open angle glaucoma [19]. Moderate myopia (-3 to -4 D), is the most common refractive error in patients of PDS and PG [22].
The CCT of PG patients was significantly thinner than PDS patients. The baseline IOP in PDS and PG patients was 21.75±8.27 mmHg and 35.85±9.61 mmHg, respectively. There was a statistically significant high baseline IOP in PG patients than in PDS patients, at presentation, (p=0.0001). Even patients with PDS and normal IOP, have been noted to have a tendency toward large spontaneous fluctuations in their IOP [23]. This diurnal IOP variability is postulated to be linked to circadian increases in aqueous outflow obstruction because of sudden pigmentary accumulations [24]. The C:D ratio of PG patients was significantly higher than PDS patients. This is because of more neuro-retinal rim thinning in PG patients. The C:D ratio changes more gradually in large discs [25].
Among both the group, PG group patients had significantly lower MD (p=0.0001) and VFI (p=0.0003). These patients can experience rapid deterioration of their visual function and often a more complicated course requiring additional surgeries [3].
Limitation(s)
The limitations of the present study were its retrospective nature, small sample size and for detection of ITDs, a standard slit-lamp examination in dark room was performed. Whereas use of an infrared imaging technique, which has demonstrated to be helpful in detecting ITDs in black patients [12,14], would have been more helpful to pick up iris defects in the Indian patients also.
Conclusion(s)
The clinical features of PDS in Indians are almost similar to that of blacks. At the time of initial diagnosis of PDS, 82.5% of the patients had Krukenberg spindle and 60% of patients (24 of 40) had PG. All forty patients had involvement of both eyes either as PDS or PG. PDS patients with normal optic disc and visual field and raised IOP, should be started prophylactic treatment. Patients with PDS and PG need to be monitored more closely. Thus, the finding of PDS in Indians should alert the ophthalmologist to look for glaucoma at the initial examination.
PDS: Pigment dispersion syndrome; PG: Pigmentary glaucoma; *Chi-square test
$unpaired t test, statistical significance level ≤0.05
BCVA: Best corrected visual acuity; CCT: Central corneal thickness; MD: Mean deviation; VFI: Visual field index; $unpaired t test, statistical significance level ≤0.05
KS: Krukenberg spindle; DP: Diffuse pigmentation; TM: Trabecular meshwork; ITD: Iris trans-illumination defect; Grd: Grade