Introduction
Dengue, a vector borne viral infection transmitted by Aedes mosquito has recently become a major public health concern in the tropical regions of the world. In addition to the two major life threatening complications- Dengue Haemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS), a variety of cardiac complications have been recognised, the most common being myocarditis.
Aim
To study and compare the quantity of different cardiac biomarkers in patients of Dengue Fever with and without myocarditis.
Materials and Methods
This was a hospital-based retrospective observational study done in a Tertiary Care Hospital, Kolkata, West Bengal, India from June 2019 to November 2019. Dengue patients with diagnosed myocarditis on day 7 of fever based on electro and echocardiogram changes of left ventricular ejection fraction less than 50% were considered as cases (n=41). Age and sex matched dengue patients with normal electro and echocardiogram changes were considered as control (n=43). After obtaining Institutional Ethics Committee Clearance, laboratory data were collected from samples coded and assayed for markers of acute cardiac myocyte damage such as total Creatine Kinase (CK), CK-Muscle Brain (CK-MB), Troponin T (Trop T) and cardiac failure biomarker N-Terminal pro Brain Natriuretic Peptide (NT-proBNP). Statistical analysis of the data was performed using Statistical Package for Social Sciences (SPSS 20).
Results
Cardiac biomarkers CK, CK-MB, Trop T and NT-proBNP levels in cases were higher compared to controls (p-value <0.05). Trop T and NT-proBNP were positively correlated to each other (r-value: 0.44). Trop T changes could also predict significantly the rise in NT-proBNP in circulation (p<0.05).
Conclusion
It reconfirmed the need of routine monitoring of cardiac biomarkers in conjunction with other cardiac function tests in early diagnosis and or management of myocarditis, a severe complication of Dengue Viral Infection (DENV).
Introduction
Dengue, a vector borne viral infection of Flaviviridae family is transmitted by Aedes mosquito has recently become a major public health concern in the tropical regions of the world. Modelling estimate indicates 390 million dengue virus infections per year, of which 96 million manifest clinically with any severe symptoms related to the disease [1]. Prevalence studies estimated that 3.9 billion people are at risk of infection with dengue viruses. Despite a risk of infection exists across 129 countries, 70% of the actual burden is in Asia [1,2]. All the serotypes of the virus can cause dengue fever, a self limiting febrile illness [3]. The sequential or secondary infection by different serotypes of dengue virus induces the two major life threatening complications- Dengue Haemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS) which is due to cross-reactive non-neutralising antibodies from previous dengue virus infection [4]. This is also characterised by cytokine mediated increased vascular permeability by T cells which destroy dengue-infected cells [5], and this syndrome in its most severe form can threaten the patient’s life [6].
Presence of viral proteins C and Non-Structural Protein 1 (NS1) in confocal microscopic examination of cultured human skeletal muscle myotubes have revealed that heart and skeletal muscles are target organs for DENV [7]. Viruses induced increase in intracellular calcium ions and interference with calcium homeostasis is responsible for altered myocardial contractility and evolution of arrhythmias. Increased calcium produces mitochondrial pore opening, cytochrome c release, caspase activation, and nuclear apoptosis in cells exposed to infection [8]. It is known fact that dengue virus has preference to infect peripheral blood and vasculature more than vital organs, particularly myocardium [9,10]. Still, a range of cardiac complications have been documented, the most common being myocarditis, although conduction defects and arrhythmias have also been reported [11,12]. Myocardial damage in dengue is hypothesised to be orchestrated by a combination of both direct virus and subsequent immune mediated mechanism [13].
To date, various studies had addressed the correlation of cardiac involvement in dengue with various biochemical and haematological parameters with diverse results [14,15]. With this background, this study was designed to evaluate and compare the biochemical changes of cardiac biomarkers like total CK, CK-MB, Trop T and cardiac failure biomarker NT-proBNP between patients of Dengue Fever with Myocarditis (DFM) as cases and age and sex matched patients of Dengue Fever without Myocarditis (DFWM) as controls.
Materials and Methods
This retrospective hospital based observational study was done in the Microbiology Department of IDBG Hospital Kolkata and Department of Biochemistry of IPGMER, Kolkata. After obtaining clearance from Institutional Ethics Committee (IDBGH/Ethics/2447) all procedures followed were in accordance with the Helsinki Declaration of 1975 that was revised in 2000. Symptomatic patients admitted in a Tertiary Care Center and fulfilling the inclusion criteria following serological testing were included in this study conducted for a period of 6 months from June 2019 to November 2019. Collection of laboratory data of all cases and controls were undertaken with history and clinical data of each patient that was recorded formally, evaluated retrospectively in present study.
Patients admitted in the IDBG, Hospital with Dengue with coronary manifestation within 18-65 years of age are taken as cases and controls are selected as ethnicity, age and sex matched having all the exclusion criteria and no obvious coronary manifestation. Being an observational study, formal sample size calculation is not done here. Keeping time and logistic constraints in mind 41 cases (DFM) and 43 controls (DFWM) have been included in this study.
Inclusion criteria: Serologically diagnosed Dengue patients with clinical features suggestive of cardiac involvement with abnormal electro and echocardiogram findings within 7 days of fever were considered for the study. ECHO findings of Left Ventricular Ejection Fraction (LVEF) of <50% was considered abnormal [16]. These patients had typical manifestations of acute viral infection such as fever, myalgia. Gradually, there was development of progressive dyspnoea one or two weeks after start of acute symptoms suggestive of possible cardiac involvement as sequelae of dengue infection [17].
Exclusion criteria: It included patients having conditions which might cause falsely elevated Trop T, CK-MB or total CK levels, such as sepsis, kidney failure or chronic kidney disease, cancer chemotherapy, pulmonary embolism, cocaine and similar drug use, injury to heart or skeletal muscle, surgery, chest trauma, asthma, malignancies, pre-existing myopathies, endocrine disorder. Moreover, cases with previous history of heart disease like arrhythmias or heart failure due to cardiac conditions other than viral myocarditis were excluded from the study [18].
Sample analysis: At the time of admission, data regarding age, sex, risk factors, onset and grade of symptoms were collected in detail according to standard hospital protocol. Study population consisted of people residing within 4 km radius from the place of study in areas of dengue epidemicity. All investigation details were documented. During stay in hospital, as part of routine clinical workout, venous blood samples were collected from each case and control after 12 hours of fasting. Blood sample was collected randomly by standard venipuncture technique into clotted vials using aseptic precautions from those of cases and controls. Complete clot formation was ensured prior to centrifugation. Serum was separated after centrifuging for 15 minutes, and was analysed for all the parameters on the same day. Dengue NS1 antigen was detected by Enzyme Linked Immunosorbant Assay (ELISA; InBios, USA). A 3rd generation IgM capture ELISA (Panbio, Abott, India) was then performed according to manufacturer’s instructions for confirmation of presence of anti-Dengue antibodies. All patients underwent Electrocardiography (ECG) of all leads along with 2-dimensional Echocardiography (ECHO). All samples were coded and assayed for markers of acute cardiac myocyte damage such as total CK, CK-MB, Trop T and cardiac failure biomarker NT-proBNP in a blind fashion by an investigator who was unaware of the subjects’ clinical status. Cardiac enzymes were estimated biochemically by automated analyser (Transasia, India) [19]. Trop T was measured using an enzyme-linked fluorescent assay (VIDAS Trop T. Ultra, bioMérieux, France) [20]. NT-proBNP levels were measured by an ELISA (VIDAS NT-proBNP, bioMérieux, France) [21]. All these data were analysed retrospectively.
Statistical Analysis
Statistical analysis of the data was performed using SPSS 20 and inferences were drawn. Comparison of continuous variables between cases and control groups were evaluated using Student’s t-test. Categorical variables were compared using Pearson’s chi-square test (χ2) test. Probability value p<0.05 was considered to be statistically significant at a confidence limit of 95%. Pearson’s correlation test was performed to establish the strength of association between continuous variables (r value). As a predictive analysis, the multiple linear regressions were used to explain the relationship between one continuous dependent variable and multiple independent variables.
Results
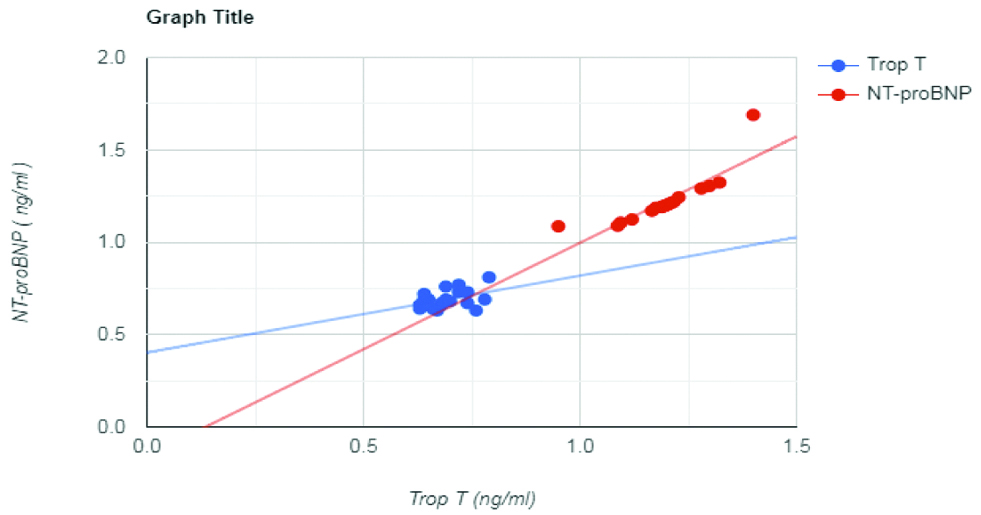
Post screening the final eligible study population comprised of 41 cases (DFM) and 43 controls (DFWM) that were age and sex matched as far as possible by Independent t-test and chi-square tests, respectively. Baseline characteristics of the entire study population and results of comparison of various cardiac biochemical parameters values between cases and controls are highlighted in [Table/Fig-1]. Presence or absence of independent risk factors such as Diabetes Mellitus, hypertension, smoking, documented viral fever within last three months prior to dengue infection was found to have no statistical bearing on development of myocarditis. Serological screening and confirmation of Dengue was completed for the entire study population within first 5 days of fever. ECHO and ECG were then performed in cases with symptoms of cardiac involvement. Progressive dyspnoea was observed in 8 cases with myocarditis within first week of Dengue fever. It had developed gradually in rest during 2nd and 3rd week of infection (n=19 and 14, respectively). Majority of patients with myocarditis had developed Grade III dyspnoea. Palpitation was complained by few cases and controls. However, only 3 cases had ECG findings of true ventricular tachycardia while the rest were non-specific. Cases had elevated levels for all the cardiac enzymes and proteins compared to controls. The differences were statistically significant. Pearson’s correlation analysis was performed to observe the change in NT-proBNP levels with respect to rise in cardiac markers. In cases, only Trop T showed positive correlation with NT-proBNP levels (r-value: 0.44) [Table/Fig-2 and 3]. A multiple regression analysis was run to predict whether NT-proBNP variation was dependant on independent factors like changes in values of total CK, CK-MB and TROP-T or not. Of all these variables only TROP-T significantly predicted NT-proBNP rise as a late effect of dengue myocarditis (p<0.05) [Table/Fig-4].
Baseline characteristics and comparison of serum levels of cardiac biomarkers in cases and controls on Day 7 of fever.
| Parameter (Mean+SD) | Dengue Fever with Myocarditis (DFM) (Cases, n=41) | Dengue Fever without Myocarditis (DFWM) (Controls, n=43) | p-value |
|---|
| Age (In years) | 48±10 | 43±12 | 0.41* |
| Sex distribution | Male | 26 | 24 | 0.47** |
| Female | 15 | 19 |
| Independent Risk Factors (Diabetes Mellitus, Hypertension, Smoking, Documented viral fever within last 3 months) | 20 (48%) | 25 (58%) | 0.38** |
| 21 (52%) | 18 (42%) |
| Days of onset of dyspnoea | <7 | 8 | NIL | |
| 7-14 | 19 | NIL | |
| >14 | 14 | NIL | |
| Grade of dyspnoea (NYHA classification) | Class I | 6 (14%) | NIL | |
| Class II | 10 (25%) | NIL | |
| Class III | 23 (56%) | NIL | |
| Class IV | 2 (5%) | NIL | |
| Palpitation | 6 | 2 | |
| Total CK (U/L) | 201±57 | 158±49 | 0.0102* |
| CK-MB (U/L) | 27±6.8 | 22±5.6 | 0.0107* |
| Trop T (ng/ml) | 0.61±0.04 | 0.07±0.01 | 0.0001* |
| NT-proBNP (125 μg/L) | 1210±25 | 192±76 | <.001* |
p-value <0.05: Significant
*Independent t-test, **Chi-square test (χ2)
NYHA: New York heart association
Correlation between the biochemical parameters in dengue fever with myocarditis.
| Sl No. | Parameters versus NT-proBNP (μg/L) | Correlation coefficient (R-value)* |
|---|
| 1 | Total CK (U/L) vs NT-proBNP (μg/L) | -0.197 |
| 2 | CK-MB (U/L) vs NT-proBNP (μg/L) | 0.174 |
| 3. | Trop T (ng/mL) vs NT-proBNP (μg/L) | 0.443 |
CK: Creatine kinase; CK-MB: Creatine kinase-muscle brain; Trop T: Troponin T; NT-BNP: N-Terminal pro brain natriuretic peptide
*Pearson’s correlation analysis
Scatter plot to correlate Trop T with NT-proBNP values.
NT-ProBNP values have been converted and expressed in terms of ng/mL
Multiple regression analysis for biochemical parameters in dengue fever with myocarditis.
| Model summary | R | R square | Adjusted R square | Std. error of the estimate | |
|---|
| 0.47 | 0.23 | 0.16 | 151.68 | |
| ANOVA | Sum of squares | df | Mean square | F | Sig. |
| Regression | 247111.28 | 3 | 82370.43 | 3.580 | 0.02* |
| Residual | 828264.31 | 36 | 23007.34 | | |
| Total | 1075375.6 | 39 | | | |
| Coefficients | Unstandardised coefficients | Standardised coefficients | t | Sig. | 95.0% Confidence interval for B |
| B | Std. error | Beta | Upper bound | Lower bound |
| (Constant) | -345.1 | 1336.19 | | -0.25 | 0.79 | -3054.94 | 2364.8 |
| Total CK | 5.12 | 7.27 | 0.191 | 0.70 | 0.48 | -9.62 | 19.87 |
| CK-MB | -16.98 | 14.56 | -0.301 | -1.17 | 0.25 | -46.52 | 12.56 |
| TROP-T | 1602.3 | 537.506 | 0.484 | 2.981 | 0.005 | 512.284 | 2692.5 |
a: Dependent Variable: NT-proBNP
b: Predictors: (Constant), Total CK, CK-MB, TROP-T
R: Multiple correlation coefficient; R2: Coefficient of determination; Adjusted R Square: Adjusts for nonsignificant predictors; Std. Error of the Estimate: Measure of the accuracy of predictions
*Analysis of variance (ANOVA), Multiple Regression Analysis
Discussion
Myocarditis is a common complication of severe dengue infection. However, data about prevalence and characterisation of myocarditis among Indian population in dengue are still lacking. Several studies have reported cardiac involvement in dengue including myocarditis and heart failure [10-12]. In 2005, an epidemic showed high incidence of myocarditis [13]. Myocarditis is an especially common cause of death in patients with severe dengue [16,17]. Prevalence of myocarditis in dengue was reported from 9% to 15% [18,22,23]. This variation in prevalence of myocarditis in dengue may result from differences in host genetic factors and type of dengue serotype circulating in a region, which may modify the resultant host inflammatory responses. Also, separate temporal patterns of circulating serotypes might predispose to particular dengue immune status that causes more severe cardiac manifestation when there is a secondary infection.
In dengue, damage to myocardial cells occurs due to direct viral invasion of the myocardium and derangement of calcium storage in the infected cells [24]. The clinical spectrum varies from nonspecific electrocardiographic abnormalities to sudden cardiac arrest [25,26]. Cardiac arrhythmia and heart failure are very common. Accordingly, dengue patients developing myocarditis have different diagnostic and laboratory performances. The treatment of viral myocarditis varies by clinical presentation.
Targeted therapy for acute heart failure is critical for recovery [27,28] as approximately 20% of those who developed dengue haemorrhagic fever have a left ventricular ejection fraction of less than 50% [29]. In dengue, a mediators like TNF-α and nitric oxide have been reported to be altered. Cytokine release activates matrix metalloproteinase which disrupts cardiac scaffolding of collagen and elastin protein causing dilated cardiomyopathy [30,31]. NT-proBNP is an established surrogate marker of heart failure and this is inversely correlated to ejection fraction. Study conducted upon 100 heart failure patients clearly showed that there exists a strong negative correlation between NT-proBNP concentration and LVEF (p<0.004). A cut-off value of 940 pg/mL for NT-proBNP predicted LVEF <30%, with a sensitivity and the specificity of 89.8% and 71.4%, respectively [32]. This is evident in study results in terms of decreased cardiac ejection fraction in cases and simultaneous high serum NT-proBNP levels compared to controls. In study on patients with myocarditis, presence of viral genomes in the myocardium like parvovirus, parvovirus B19, HHV-6 and Epstein-Barr virus, were associated with an increased hs-TnT level compared to those with myocarditis but without evidence of viral genomes [33]. In the present study, serum Trop T levels in cases were significantly raised in comparison to controls. Serum levels of cardiac enzymes CK and CK-MB were found to be significantly elevated in cases with respect to controls. These findings are in contrast to data obtained by other workers, who reported that during the course of myocarditis, the laboratory markers of myocardial cell damage, such as CK and CK-MB levels were often within the normal range [34,35]. But the present study finding is in accordance with some other researchers [36,37]. These findings are important because raised CK, CK-MB and Trop T levels suggest injury to myocardial tissue. Yacoub S et al., stated that cardiac biomarker elevation and marked changes on ECHO or ECG are evidence of widespread myocyte infection and damage in fulminant cases of dengue myocarditis [38]. Among dengue patients with elevated cardiac biomarker Troponin I, Miranda CH et al., had reported that 10% (8 patients) patients presented with clinical manifestations suggestive of cardiac involvement, such as acute heart failure in 4 patients, chest pain in 3 patients, and hypotension and shock in 3 patients [30]. Findings from the study suggest that only a small number of dilated cardiomyopathy cases (n=3) had developed ventricular tachyarrhythmia. Among the cardiac biomarkers only Trop T had some correlation with NT-Pro BNP [Table/Fig-3]. This suggests importance of Trop T estimation over prescribing a total cardiac profile testing in suspected viral myocarditis cases. Absence of a strong linear relationship makes the authors believe that the timing of the two events of myocardial injury and the resultant heart failure varies in individuals. Trop T is showing as significant predictor for the detection of myocardial involvement in dengue as depicted in multivariate regression analysis. Trop T assays are well suited for early diagnosis of myocarditis [39].
In a middle aged adult progressive dysponea had developed only after two weeks of serological confirmation. The ejection fraction of these cases is reported to become normal in due course of time [40]. Diagnoses of cardiac involvement in viral disease are still based on elevated biomarkers of cardiac injury along with ECG and ECHO findings [41]. Established role of measurement of anti-heart antibodies or endomyocardial biopsy on a routine basis for diagnosis and evaluation is lacking. A small number of cases present with fulminant myocarditis causing cardiogenic shock and resulting in multiorgan failure [42]. Recognition of this phenomenon is of utmost importance as aggressive management is potentially life-saving [43].
Limitation(s)
In the present study, some methodological limitations exist. The study was conducted in a Tertiary Care Hospital and is primarily a cross sectional study. However, in India, most people visit district, sub divisional and lower-tier hospitals for treatment. This particular study population comprised of only Dengue infected persons. Other patients with a history of pre-existing heart disease with previous echocardiographic abnormality were excluded from the study. Hence, results of this study might not reflect the true picture of the population as a whole. In addition, subjects were not divided according to Dengue serotypes because it is not definite that infection by a particular serotype predispose to myocarditis. Lastly, variations in age groups, co-morbidities, and study design might be additional factors causing separate manifestations and incidences but it was the limitation of the study.
Conclusion(s)
This study demonstrates diagnostic characteristics of dengue associated myocarditis. Data from the present study shows that cardiac biomarkers are elevated in dengue myocarditis and hence should be measured routinely in conjunction with other function tests in early diagnosis and management. Estimation of Trop T is reliable point of care testing to diagnose myocarditis among Dengue patients, though the results should be substantiated with further research in a larger multicentric population.
p-value <0.05: Significant
*Independent t-test, **Chi-square test (χ2)
NYHA: New York heart association
CK: Creatine kinase; CK-MB: Creatine kinase-muscle brain; Trop T: Troponin T; NT-BNP: N-Terminal pro brain natriuretic peptide
*Pearson’s correlation analysis
a: Dependent Variable: NT-proBNP
b: Predictors: (Constant), Total CK, CK-MB, TROP-T
R: Multiple correlation coefficient; R2: Coefficient of determination; Adjusted R Square: Adjusts for nonsignificant predictors; Std. Error of the Estimate: Measure of the accuracy of predictions
*Analysis of variance (ANOVA), Multiple Regression Analysis
[1]. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, The global distribution and burden of dengueNature 2013 496(7446):504-07.10.1038/nature1206023563266 [Google Scholar] [CrossRef] [PubMed]
[2]. Brady OJ, Brady OJ, Bhatt S, Messina JP, Brownstein JS, Hoen AJ, Refining the global spatial limits of dengue virus transmission by evidence-based consensusNeglected Tropical Diseases 2012 6(8):176010.1371/journal.pntd.000176022880140 [Google Scholar] [CrossRef] [PubMed]
[3]. Shrivastava S, Tiraki D, Diwan A, Lalwani SK, Modak M, Mishra AC, Co-circulation of all the four dengue virus serotypes and detection of a novel clade of DENV-4 (genotype I) virus in Pune, India during 2016 seasonPLoS ONE 2018 13(2):e019267210.1371/journal.pone.019267229470509 [Google Scholar] [CrossRef] [PubMed]
[4]. Pruthvi D, Shashikala P, Shenoy V, Evaluation of platelet count in dengue fever along with seasonal variation of dengue infectionJournal of Blood Disorders & Transfusion 2012 3:01-04.10.4172/2155-9864.1000128 [Google Scholar] [CrossRef]
[5]. Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severityThe Journal of Infectious Diseases 2000 181(1):02-09.10.1086/31521510608744 [Google Scholar] [CrossRef] [PubMed]
[6]. Halstead SB, DengueLancet 2007 370(9599):1644-52.10.1016/S0140-6736(07)61687-0 [Google Scholar] [CrossRef]
[7]. WHO: Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Geneva. 1997;24-30 [Google Scholar]
[8]. Szalai G, Krishnamurthy R, Hajnoczky G, Apoptosis driven by IP(3)-linked mitochondrial calcium signalsEMBO J 1999 18:6349-61.10.1093/emboj/18.22.634910562547 [Google Scholar] [CrossRef] [PubMed]
[9]. Rigau-Pérez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV, Dengue and dengue haemorrhagic feverLancet 1998 352(9132):971-77.10.1016/S0140-6736(97)12483-7 [Google Scholar] [CrossRef]
[10]. WHO: Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Geneva. 1997;24-30 [Google Scholar]
[11]. Kanakaratne N, Wahala WM, Messer WB, Tissera HA, Shahani A, Abeysinghe N, Severe dengue epidemics in Sri Lanka, 2003-2006Emerg Infect Dis 2009 15:192-99.10.3201/eid1502.08092619193262 [Google Scholar] [CrossRef] [PubMed]
[12]. Kabra SK, Juneja R, Jain Y, Madhulika Jain Y, Singhal T, Dar L, Myocardial dysfunction in children with dengue haemorrhagic feverNat Med Jr Ind 1998 11(2):59-61. [Google Scholar]
[13]. Promphan W, Sopontammarak S, Pruekprasert P, Kajornwattanakul W, Kongpattanayothin A, Dengue MyocarditisSoutheast Asian Jr Tropical Med Public Health 2004 35(3):611-13. [Google Scholar]
[14]. Azin FRFG, Gonçalves RP, Pitombeira MHS, Lima DM, Branco IC, Dengue: Profile of hematological and biochemical dynamicsRev Bras Hematol Hemoter 2012 34(1):36-41.10.5581/1516-8484.2012001223049382 [Google Scholar] [CrossRef] [PubMed]
[15]. Ferede G, Tiruneh M, Abate E, Wondimeneh Y, Gadisa E, Howe R, A study of clinical, hematological, and biochemical profiles of patients with dengue viral infections in Northwest Ethiopia: Implications for patient managementBMC Infect Dis 2018 18:61610.1186/s12879-018-3557-z [Google Scholar] [CrossRef]
[16]. Fonseca BAL, Fonseca SN, Dengue virus infectionsCurr Opin Pediatr 2002 14:67-71.10.1097/00008480-200202000-0001211880737 [Google Scholar] [CrossRef] [PubMed]
[17]. Sheetal S, Jacob E, A study on the cardiac manifestations of dengueJ Assoc Physicians India 2016 64:30-34. [Google Scholar]
[18]. Al-Hadi HA, Fox KA, Cardiac markers in the early diagnosis and management of patients with acute coronary syndromeSultan Qaboos Univ Med J 2009 9(3):231-46. [Google Scholar]
[19]. Henderson AR, Donald WM, Enzymes Tietz Fundamentals of Clinical Chemistry, 5th Ed., Burtis, C.A. & Ashwood, E.R. (W.B. Saunders eds. Philadelphia USA) 2001 :352 [Google Scholar]
[20]. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010Lancet 2012 380(9859):2059-128.10.1016/S0140-6736(12)61728-0 [Google Scholar] [CrossRef]
[21]. Januzzi JL, Kimmenade RV, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP StudyEuropean Heart Jour 2006 27(3):330-37.10.1093/eurheartj/ehi63116293638 [Google Scholar] [CrossRef] [PubMed]
[22]. Hober D, Delannoy AS, Benyoucef S, De Groote D, Wattré P, High levels of sTNFRp75 and TNF alpha in dengue-infected patientsMicrobiology Immunology 1996 40(8):569-73.10.1111/j.1348-0421.1996.tb01110.x8887351 [Google Scholar] [CrossRef] [PubMed]
[23]. Miranda CH, Borges Mde C, Schmidt A, Pazin-Filho A, Rossi MA, Ramos SG, A case presentation of a fatal dengue myocarditis showing evidence for dengue virus-induced lesionEur Heart J Acute Cardiovasc Care 2013 2(2):127-30.10.1177/204887261347588924222821 [Google Scholar] [CrossRef] [PubMed]
[24]. Marques N, Gan VC, Leo YS, Dengue myocarditis in Singapore: Two case reportsInfection 2013 41(3):709-14.10.1007/s15010-012-0392-923277366 [Google Scholar] [CrossRef] [PubMed]
[25]. Kularatne SA, Pathirage MM, Kumarasiri PV, Gunasena S, Mahindawanse SI, Cardiac complications of a dengue fever outbreak in Sri Lanka, 2005Trans R Soc Trop Med Hyg 2007 101:804-08.10.1016/j.trstmh.2007.02.02117428513 [Google Scholar] [CrossRef] [PubMed]
[26]. Singhi S, Jayashree M, Dengue shock syndrome: At the heart of the issuePediatr Crit Care Med 2007 8:583-84.10.1097/01.PCC.0000288677.56953.2917989564 [Google Scholar] [CrossRef] [PubMed]
[27]. Wiwanitkit V, Dengue myocarditis, rare but not fatal manifestationInt J Cardiol 2006 112:12210.1016/j.ijcard.2005.09.02716307808 [Google Scholar] [CrossRef] [PubMed]
[28]. Neeraja M, Lakshmi V, Teja VD, VanjariLavanya V, Priyanka EN, Subhada K, Unusual and rare manifestations of dengue during a dengue outbreak in a tertiary care hospital in South IndiaArch Virol 2014 159:1567-73.10.1007/s00705-014-2010-x24510171 [Google Scholar] [CrossRef] [PubMed]
[29]. Salgado DM, Eltit JM, Mansfield K, Panqueba C, Heart and skeletal muscle are targets of dengue virus infectionPediatr Infect Dis J 2010 29:238-42.10.1097/INF.0b013e3181bc3c5b20032806 [Google Scholar] [CrossRef] [PubMed]
[30]. Miranda CH, Borges Mde C, Matsuno AK, Vilar FC, Gali LG, Volpe GJ, Evaluation of cardiac involvement during dengue viral infectionClin Infect Dis 2013 57:812-19.10.1093/cid/cit40323784923 [Google Scholar] [CrossRef] [PubMed]
[31]. Sengupta SP, Nugurwar A, Jaju R, Khandheria BK, Left ventricular myocardial performance in patients with dengue hemorrhagic fever and thrombocytopenia as assessed by two-dimensional speckle tracking echocardiographyIndian Heart J 2013 65(3):276-82.10.1016/j.ihj.2013.04.01723809381 [Google Scholar] [CrossRef] [PubMed]
[32]. Koç M, Bozkurt A, Yildiray-Sahin D, Unal I, Acartürk E, Cutoff values of NT-proBNP for the prediction of low functional capacity, decreased ejection fraction and cardiovascular events in patients with heart failureCardiol J 2009 16(1):43-49. [Google Scholar]
[33]. Ukena C, Kindermann M, Mahfoud F, Geisel J, Lepper PM, Kandolf R, Diagnostic and prognostic validity of different biomarkers in patients with suspected myocarditisClin Res Cardiol 2014 103(9):743-51.10.1007/s00392-014-0709-z24781421 [Google Scholar] [CrossRef] [PubMed]
[34]. Yacoub S, Griffiths A, Chau TT, Simmons CP, Wills B, Hien TT, Cardiac function in Vietnamese patients with different dengue severity gradesCrit Care Med 2012 40(2):477-83.10.1097/CCM.0b013e318232d96621946658 [Google Scholar] [CrossRef] [PubMed]
[35]. Kohli U, Sahu J, Lodha R, Agarwal N, Ray R, Invasive nosocomial aspergillosis associated with heart failure and complete heart block following recovery from dengue shock syndromePediatr Crit Care Med 2007 8:389-91.10.1097/01.PCC.0000269397.95479.3C17545933 [Google Scholar] [CrossRef] [PubMed]
[36]. La-Orkhun V, Supachokchaiwattana P, Lertsapcharoen P, Khongphatthanayothin A, Spectrum of cardiac rhythm abnormalities and heart rate variability during the convalescent stage of dengue virus infection: A Holter studyAnn Tropic Paediatr 2011 31:123-28.10.1179/1465328111Y.000000000821575316 [Google Scholar] [CrossRef] [PubMed]
[37]. Bethell DB, Gamble J, Pham PL, Nguyen MD, Tran TH, Ha TH, Non-invasive Measurement of microvascular leakage in patients of dengue haemorrhagic feverClin Infect Dis 2001 32:243-53.10.1086/31845311170914 [Google Scholar] [CrossRef] [PubMed]
[38]. Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B, Cardiovascular manifestations of the emerging dengue pandemicNat Rev Cardiol 2014 11:335-45.10.1038/nrcardio.2014.4024710495 [Google Scholar] [CrossRef] [PubMed]
[39]. Lauer B, Niederau C, Kuhl U, Schannwell M, Pauschinger M, Strauer BE, Cardiac troponin T in patients with clinically suspected myocarditisJ Am Coll Cardiol 1997 30(5):1354-59.10.1016/S0735-1097(97)00317-3 [Google Scholar] [CrossRef]
[40]. Smith SC, Ladenson JH, Mason JW, Jaffe AS, Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlatesCirculation 1997 95(1):163-68.10.1161/01.CIR.95.1.1638994432 [Google Scholar] [CrossRef] [PubMed]
[41]. Lee C, Teo C, Low AF, Fulminant dengue myocarditis masquerading as acute myocardial infarctionInt Jr Cardiol 2009 136(3):69-71.10.1016/j.ijcard.2008.05.02318701172 [Google Scholar] [CrossRef] [PubMed]
[42]. Patra S, Bhardwaj G, Manohar JS, Srinivasa KH, Kharge J, Manjunath CN, Acute myocardial infarction being the presentation of dengue myocarditisJ Cardiovasc Dis Res 2013 4(2):159-61.10.1016/j.jcdr.2013.03.00124027378 [Google Scholar] [CrossRef] [PubMed]
[43]. Lakdawala NK, Stevenson LW, Loscalzo J, Cardiomyopathy and myocarditis. In: Jameson JL, Kasper DL, Longo DL, Fauci AS, Hauser SL, Loscalzo J, edsHarrison’s principles of internal medicine 2015 20 edNew YorkMcGraw-Hill:1783-85. [Google Scholar]