The wounds of hospitalised patients may result from surgery, pressure ulcers, and diabetic ulcers, etc. The wound can be classified as clean wounds or contaminated based on their degree of contamination [10]. The clean wounds have minimal risk of infection, i.e., lipoma excision, thyroidectomy, simple herniorrhaphy, and lumpectomy limited to 1-2% risk of infection. The contaminated wounds have a high rate of infection (15-20% infection rate), and it occurs when there is a major breach in aseptic conditions even without entry into the abscess cavity. Dirty or Infected wounds have a very high risk of infection (>40% infection rate). Dirty wounds appear as grossly infected with pus with entry into an abscess cavity such as in gangrenous bowel [11]. In hospital environments, wounds are likely to get colonised with multidrug-resistant organisms, especially MRSA. Bacterial colonisation of wounds can increase wound severity and interfere with healing. Surveillance of the pattern of Staphylococcal infections and their antibiogram in a hospital environment is therefore warranted, especially in postoperative surgical wounds, which may be a source of cross-contamination by MRSA and Coagulase-Negative Staphylococcus species (CoNS) [12].
Materials and Methods
The present cross-sectional study was carried out after the approval from Sumandeep Vidyapeeth Institutional Ethics Committee (Ref. No.- SVIEC/ON/Medi/PhD/17007) in August 2017 and data collection was done for two years (from August 2017 to July 2019). The clinical history of the patient having staphylococcal infection was taken from the MRD section of the study place. As in the present study, there was no direct involvement of human, animal, or their body parts, hence consent from the patients were not required.
Inclusion criteria: The pus samples collected from the infected chronic wounds by aspiration in sterile syringes or by sterile swab received in the Department of Microbiology were included in the present study.
Exclusion criteria: Repeat sample from the same patients were excluded from the study.
Study procedure: The specimens were inoculated on 5% sheep blood agar and MacConkey Agar and incubated at 37°C for 24 hours. Subsequently, a smear made from the direct specimen and stained with gram stain and examined under oil immersion lens, and the primary report was sent to the clinician for initial treatment. After 24 hours of incubation of previously inoculated clinical specimens, isolated colonies were taken to make a smear and stained with gram stain to rule out Gram-positive cocci arranged in clusters. Confirmed gram-positive cocci were further subjected to the Catalase test to differentiate staphylococci from streptococci. The catalase-positive isolated colonies were tested for coagulase production to categorise Staphylococcus aureus and CoNS species. The tube coagulase test was done, the tube was incubated at 37°C for 4 hours if the clot was not observed at the end of four hours; the tube was further incubated at room temperature and read after 18-24 hours. The Catalase-positive and Coagulase-negative isolates were further subjected to antimicrobial susceptibility testing by using Novobiocin (5μg), Polymyxin B (300U) disc, and Urease activity as per the standard procedure for the speciation of CoNS species [15,16].
Furthermore, a well-isolated colony was taken and suspended in peptone water and incubated at 37°C for four hours, the bacterial suspension was compared with 0.5 McFarland turbidity standard, a comparison was corrected by using the addition of peptone water or further incubation. The 0.5 bacterial suspensions were used for antimicrobial susceptibility testing and biochemical test as per standard microbiological procedure and Clinical Laboratory Standards Institute (CLSI) guidelines [17].
The erythromycin-resistant and clindamycin sensitive isolates were further subjected to D-test to rule out inducible clindamycin resistant strains of Staphylococcus species.
D-test: (disc diffusion test/disc approximation test): In D-test, erythromycin (15 μg) disc was placed at a distance of 15 mm (edge to edge) from clindamycin (2 μg) disc on Muller Hinton agar plate previously inoculated with 0.5 McFarland bacterial suspensions and incubated at 370C, flattening “D shaped” zone of inhibition around clindamycin in the area between two disc, indicated inducible clindamycin resistance [17,18].
Statistical Analysis
It was done by using International Business Machines (IBM) Statistical Package for the Social Sciences (SPSS) (20 version) software. Frequencies and percentages were calculated for all the parameters. Non-parametric test was run by selecting one sample, which automatically compares observed data to hypothesised using the Chi-square test to calculate the p-value (CI-95%) and Kolmogorov-Smirnov was used to calculate average age means and Standard Deviation (SD).
Results
The wound infection was separately evaluated from the SSTIs because of the criteria considering the wound infection like open wounds, closed wounds, contusion/bruise, laceration, avulsions, punctures, perforating and penetrating wounds. A total of 159/421 (37.7%) pus samples were received from wound infections which were further evaluated for Staphylococcal species. Of which, 142/159 (89.3%) were Staphylococcus aureus and 17/159 (10.7%) were Staphylococcus epidermidis.
Demographic data of Staphylococcal wound infection is presented in [Table/Fig-1]. The majority of the patients were male 87/159 (54.7%) and female were 72/159 (45.3%). All the infected wound specimens were procured from hospitalised patients 159 (100%) those who are admitted for diabetic foot (n=29), postoperative surgical site infection (n=08), and mechanical trauma leads to contusion (closed wound, n=07) and laceration (open wound, n=115). The patients were from the age group of 1 to 80 years and the average mean age of the patients was 44.10±17.25 years (p-value-0.013) [Table/Fig-2].
Demographic data of staphylococcal wound infection.
| Demographic data | N | Percent | Bootstrap for Percent |
|---|
| Bias | Sth. Error | 95% CI |
|---|
| Lower | Upper |
|---|
| Male | 87 | 54.7 | 0.1 | 4.3 | 45.3 | 64.2 |
| Female | 72 | 45.3 | -0.1 | 4.3 | 35.8 | 54.7 |
| In-patient | 159 | 100.0 | 0.0 | 0.0 | 100.0 | 100.0 |
| Out-patient | 0 | 0 | 0 | 0 | 0 | 0 |
| Statistical test- One sample Chi-square test (p-value <0.005, 95% CI) |
| Age groups of the Patients |
| Age group (Years) | Frequency | Percent |
| 01-10 | 06 | 3.77 |
| 11-20 | 13 | 8.17 |
| 21-30 | 22 | 13.84 |
| 31-40 | 30 | 18.87 |
| 41-50 | 20 | 12.58 |
| 51-60 | 32 | 20.13 |
| 61-70 | 35 | 22.01 |
| 71-80 | 01 | 0.63 |
| Total | 159 | 100 |
| Statistical test- Kolmogorov-Smirnov (p-value <0.013, 95% CI) |
Average mean age of the patients with wound infection.
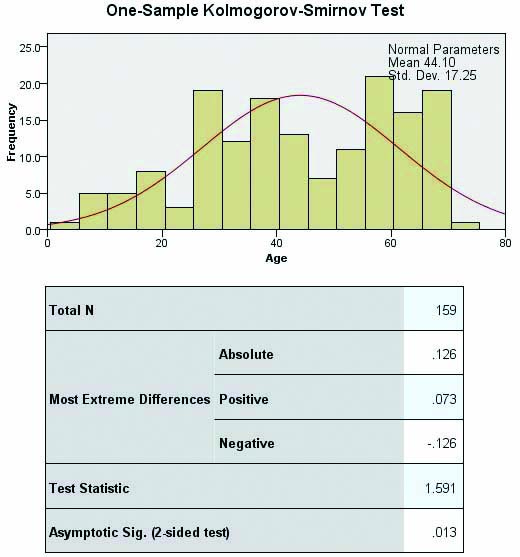
Clinical data of Staphylococcal wound infection is presented in [Table/Fig-3]. All the clinical specimens were from surgical Departments i.e., Orthopaedic 98/159 (61.6%) and Surgery 61/159 (38.4%). The majority of the patients were of diabetes 29/159 (18.2%) followed by hypertension 11/159 (7%). A prior history of antibiotic therapy relevant to wound infection was also taken [Table/Fig-3].
Clinical data of the patients of Staphylococcal wound infection.
| Clinical data of the patients | Frequency | Percent |
|---|
| Clinical Departments | Orthopaedics | 98 | 61.6 |
| Surgery | 61 | 38.4 |
| Clinical specimen | Pus swab/aspiration | 159 | 100 |
| Co-morbid conditions | Diabetic mellitus | 29 | 18.2 |
| Hypertension | 11 | 7.0 |
| None | 119 | 74.8 |
| History of prior antimicrobial therapy | Cefuroxime | 22 | 13.8 |
| Amoxyclav | 18 | 11.3 |
| Cefotaxime | 13 | 8.2 |
| Piperacillin-Tazobactam | 8 | 5.0 |
| Levofloxacin | 7 | 4.4 |
| Ofloxacin | 2 | 1.3 |
| Not given | 89 | 56.0 |
Results of Antibiotic Sensitivity Testing (AST) of Staphylococcus spp. of wound infection is depicted in [Table/Fig-4]. The antimicrobial susceptibility results are always under the influence of prior antimicrobial therapy, it is noted that patients are having exposure to baseline drugs and it might be due to the cost of the regimen, hence the staphylococcal species remain susceptible to second-line drugs (expensive). A total of 100/159 (62.9%) MRSA strains were multidrug-resistant [Table/Fig-4], all the isolates of Staphylococcus epidermidis were sensitive to methicillin (MSSE) [Table/Fig-5].
Antimicrobial susceptibility of Staphylococcus species of wound infection.
| Antimicrobial agents | Sensitive | Resistant |
|---|
| Frequency | Percent | Frequency | Percent |
|---|
| Penicillin | 0 | 0 | 159 | 100 |
| Erythromycin | 17 | 10.7 | 142 | 89.3 |
| Gentamicin | 27 | 17 | 132 | 83 |
| Ofloxacin | 28 | 17.6 | 131 | 82.4 |
| Chloramphenicol | 37 | 23.3 | 122 | 76.7 |
| Trimethoprim/Sulfamethoxazole | 45 | 28.3 | 114 | 71.7 |
| Cefoxitin (MIC) | 59 | 37.1 | 100 | 62.9 |
| Tetracycline | 58 | 36.5 | 101 | 63.5 |
| Clindamycin | 108 | 67.9 | 51 | 32.1 |
| Rifampin | 159 | 100 | 0 | 0 |
| Linezolid | 159 | 100 | 0 | 0 |
| Vancomycin (MIC) | 159 | 100 | 0 | 0 |
| Ceftaroline (MIC) | 159 | 100 | 0 | 0 |
Methicillin resistant strains of Staphylococcus species of wound infection.
| Methicillin resistance | Frequency | Percent | Bootstrap for Percent |
|---|
| Bias | Std. Error | 95% CI |
|---|
| Lower | Upper |
|---|
| MRSA | 100 | 62.9 | 0 | 3.4 | 57.2 | 69.2 |
| MSSA | 42 | 26.4 | -0.1 | 3.4 | 20.1 | 33.3 |
| MRSE | 0 | 0 | 0 | 0 | 0 | 0 |
| MSSE | 17 | 10.7 | 0.1 | 2.3 | 6.9 | 15.1 |
| Total | 159 | 100 | 0 | 0 | 100 | 100 |
MRSA: Methicillin resistant Staphylococcus aureus; MSSA: Methicillin sensitive Staphylococcus aureus; MRSE: Methicillin resistant Staphylococcus epidermidis; MSSE: Methicillin sensitive staphylococcus epidermidis; Statistical test: One sample Chi-square test (p-value <0.005, 95% CI)
Inducible clindamycin resistance in Staphylococcus species of wound infection is shown in [Table/Fig-6]. Out of 159 Staphylococcus species isolated from wound infections, 142/159 (89.3%) were erythromycin-resistant; these were further subjected to D-test to rule out inducible clindamycin resistant strains of Staphylococcus species. Inducible clindamycin resistant strains among wound infection were 58/159 (36.5%).
Inducible clindamycin resistance in Staphylococcus species of wound infection.
| Resistant phenotypes | Frequency | Percent | Bootstrap for percent |
|---|
| Bias | Std. Error | 95% CI |
|---|
| Lower | Upper |
|---|
| Erythromycin (S) | 17 | 10.7 | 0 | 2.4 | 6.3 | 17.0 |
| cMLSb (E-R, CD-R) | 51 | 32.1 | -0.3 | 3.7 | 24.5 | 39.0 |
| iMLSb (E-R, CD-S) | 58 | 36.5 | 0.3 | 3.6 | 29.6 | 45.3 |
| MSb (E-R, CD-S) | 33 | 20.8 | 0 | 3.3 | 14.5 | 27.7 |
| Total | 159 | 100 | 0 | 0 | 100 | 100 |
cMLSb: Constitutive MLSb phenotype, iMLSb: Inducible MLSb phenotype; MSb: macrolide Streptogramin phenotype; E-R: Erythromycin resistant; CD-S: Clindamycin sensitive CD-R: Clindamycin resistant; Statistical test: One sample Chi-square test (p-value <0.005, 95% CI)
Discussion
It is defined as the injury to the living tissue caused by different agents with various degrees of severity from a minor wound to a severe wound that can damage the whole organ, tissue, or cell. There are different agents to cause tissue injury/wound like Mechanical agents, Chemical agents, Radiant agents, Biological agents (pathogenic organisms) [19].
Infections by Staphylococcus species are often associated with wounds, especially in hospitalised patients. The wounds of hospitalised patients may result from community-acquired injuries, after surgery, pressure ulcers, and diabetic ulcers. Wound infections can result in recurrent hospitalisation [12,20]. The wound serves as a major factor for colonisation with methicillin resistant strains of Staphylococcus aureus, and it increases the severity of the infection [7,12].
In the present study, 159/421 (37.7%) Staphylococcal species were isolated from wound infection; out of which 142/421 (89.3%) were Staphylococcus aureus and 17/421 (10.7%) Staphylococcus epidermidis, these were further evaluated for the significant association of co-morbid conditions with wound infections. The average age of the patients was 44.10±17.25 years.
All the infected wound samples were taken from hospitalised patients admitted for diabetic foot, mechanical trauma (contusion), open wound (laceration), and postoperative surgical site infection.
Staphylococcus species are normally present on the skin, anterior nares, axilla, and groin areas that invade already existing wound that leads to infection and related clinical implications. Prevalence of Staphylococcal wound infections varies from the different geographical areas, as it is influenced by occupation, socio-economic background, and host immune response. Comparison of prevalence of Staphylococcus species in wound infections is compared with other studies which is represented in [Table/Fig-7] [21-26].
Comparison of prevalence of Staphylococcus species in wound infections [21-26].
| Different studies (Year) | S.aureus | CoNS |
|---|
| N | % | N | % |
|---|
| Sewunet T et al., (2013) [21] | - | - | 15/50 | 30 |
| Mama M et al., (2014) [22] | - | - | 21/145 | 14.48 |
| Al Tayyar IA et al., (2015) [23] | - | - | 14/223 | 6.3 |
| Harshan KH et al., (2015) [24] | 205/620 | 30 | - | - |
| Nanthini Devi P et al., (2017) [25] | 63/196 | 32.14 | - | - |
| Bora P et al., (2018) [26] | - | - | 52/120 | 43.3 |
| Present study (2020) | 142/421 | 33.72 | 17/421 | 4.0 |
The Staphylococcus species were further evaluated for antimicrobial susceptibility testing which shows that all the isolates were resistant to penicillin; however, all the isolates were 100% sensitive to rifampin, linezolid, vancomycin, and ceftaroline. These results are indicating that the patients had low exposure to second-line antimicrobial agents hence the infection can easily be treatable in this region.
Methicillin resistant strains of Staphylococcus species among wound infections were 62.9% while all the isolates of CoNS (S. epidermidis) were 10.7% susceptible to methicillin. This is per a study carried out by Šiširak M et al., antimicrobial susceptibility testing showed that 73% of MRSA isolates were with the same antibiotic sensitivity pattern and these were sensitive only to vancomycin [14].
In this study, inducible clindamycin resistant strains among wound infection were also observed in 36.5% strains, indicating a high prevalence of such strains in wound infection that should be ruled out on a routine basis to avoid treatment failure. In a study by Hatkar SS et al., the inducible clindamycin-resistant strains of Staphylococcus aureus were 46 (26.13%) and among them, 42 (91.3%) were MRSA stains [18].
Wound infection because of Staphylococcus can be an important cause of sepsis and its consequences. Of 55 patients studied of SSTIs leading to sepsis by Lakhani Som J et al., 29 had wound infection as a cause of sepsis, and Staphylococcus aureus was the commonest organism. Diabetes mellitus, yellow pus discharge unhealthy granulation tissue with slough, depth, and size of the wound was important risk factors in this study [27]. In the present study, diabetes was found to be the most significant host factor/co-morbid condition. The antimicrobial susceptibility of the isolated strain is the only key to recover the wound and reduce the healthcare cost burden.
Limitation(s)
As total eight patients was of postoperative surgical site infections, the screening of source of these infections was (hospital-acquired) not done.
Conclusion(s)
The patients suffering from diabetes are more prone to get infected with Staphylococcus species which leads to uncomplicated infection and the emergence of MRSA strains has left very few therapeutic alternatives to treat wound infection colonised with Staphylococcal species. Staphylococcal wound infections should be carefully treated to prevent microbial spread especially in immunecompromised patients, better patient recovery, and reducing healthcare costs.
MRSA: Methicillin resistant Staphylococcus aureus; MSSA: Methicillin sensitive Staphylococcus aureus; MRSE: Methicillin resistant Staphylococcus epidermidis; MSSE: Methicillin sensitive staphylococcus epidermidis; Statistical test: One sample Chi-square test (p-value <0.005, 95% CI)
cMLSb: Constitutive MLSb phenotype, iMLSb: Inducible MLSb phenotype; MSb: macrolide Streptogramin phenotype; E-R: Erythromycin resistant; CD-S: Clindamycin sensitive CD-R: Clindamycin resistant; Statistical test: One sample Chi-square test (p-value <0.005, 95% CI)