Introduction
The first case of coronavirus disease 19 (COVID-19) was reported in the Wuhan province of China on 31st December 2019 [1]. COVID-19 was declared as a pandemic by World Health Organization (WHO) on March 19, 2020 (epidemiological definition of pandemic is affecting more than 1,00,000 population in more than a 100 countries) [2]. Currently, there are 10 million cases in the world. In India, we have more than five million cases (approximately, 72% cases are cured) [3].
The COVID-19 has spread its wings all around mother earth and has brought life to a standstill. Some are lucky to just get away with a mild flu-like illness, while some succumb to the disease in spite of being on the ventilator in Intensive Care Units (ICU). COVID pandemic has led to a worldwide research to identify the people who are at more risk for developing the infection, increasing severity and mortality.
There are approximately 30 million confirmed cases globally. Every day the counts keep increasing and the latest counts can be found on the World Health Organisation (WHO) and European Centre for Diseases Prevention and Control websites. A link developed by the John Hopkins University shows the confirmed cases on the world map [4]. Cases have been reported from all the continents of the world except Antarctica. The cases that have been diagnosed and reported are an underestimate of the actual COVID-19 burden. According to seroprevalence surveys of Europe and United states, the actual exposure rate is 10 times more than the reported numbers [5,6]. In this systematic review, it was aimed to evaluate the effect of comorbidities on COVID-19 outcomes.
Materials and Methods
Search Methodology
A systematic review was planned according to PRISMA guidelines. Literature search was conducted across various databases which included Pubmed, Web of Science, Embase, Scopus and Cochrane library. All articles published between November 1, 2019 to July 31, 2020 were searched. Keywords or MeSH used were “COVID-19” or “SARS CoV 2” or Coronavirus disease 19 and “comorbidities” or “risk factors”. Individual risk factors were also used as key words. Such as “Diabetes”, Hypertension”, “Chronic kidney disease”, “Elderly”, “Cardiovascular disease”, “Obesity”, “Malignancy”, “Lung disorders”. The citations were copied to end note. Two researchers performed the literature search independently to prevent missing any valid studies, the references of the selected articles.
Inclusion criteria:
Randomised and non-randomised controlled trials, cohort studies, case-control studies, cross-sectional studies and case series were included,
Studies including patients with major and common comorbidities such as diabetes, hypertension, obesity, Chronic Kidney Disease (CKD), cardiovascular disease, malignancies and preexisting lung disorders,
COVID-19 should be diagnosed by Reverse Transcription Polymerase Chain Reaction (RT-PCR),
Only articles in English were considered.
Exclusion criteria:
News articles, editorials, thesis, books, case reports and clinical experience
Genetically related Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus studies
Data collection: Data were extracted independently by both the investigators. Any dispute was solved by mutual consensus. The following data of the study was documented in Microsoft excel sheet: first author, place of study, number of participants, comorbidities, result of the effect of comorbidities.
Quality assessment: The quality of the methods used in each article was assessed two independent reviewers. The criteria evaluated by the quality assessment included clear presentation of inclusion/exclusion criteria, patient/clinical information, reliable testing for disease confirmation and inclusion of appropriate sample population. The quality assessment used was based on the Joanna Briggs Institute Critical Appraisal guidelines [7].
Results
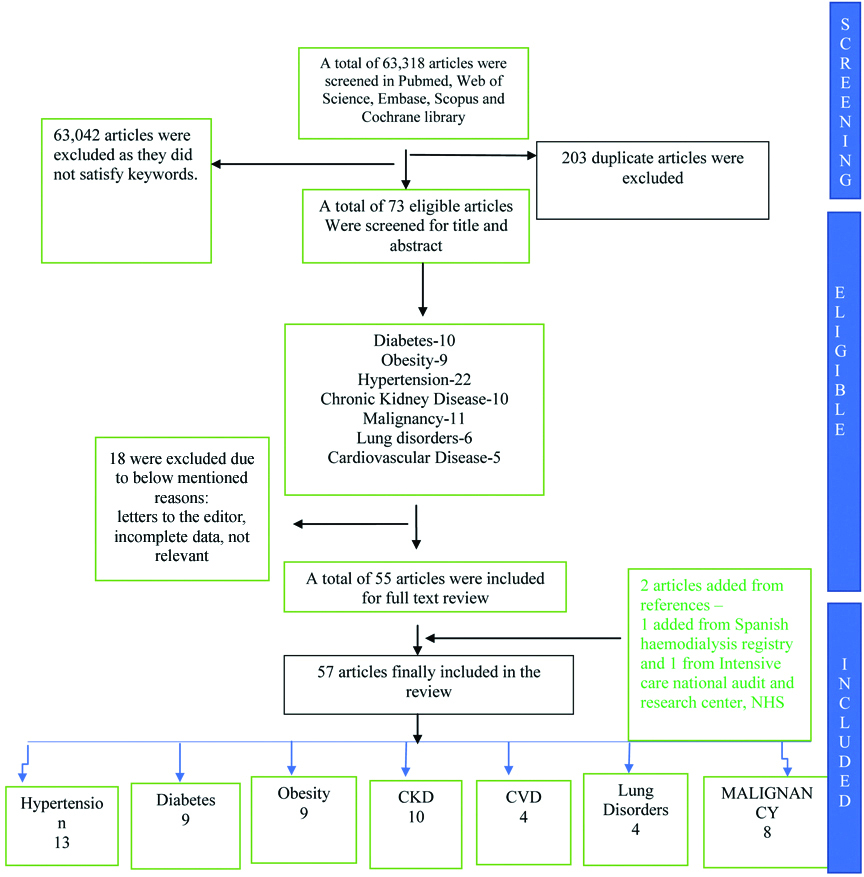
With the use of MeSH terms, 276 articles were found. A total of 203 articles were duplicated which were removed and thereafter, 73 articles were screened for title and abstract. Irrelevant articles and those with incomplete data, conference abstracts were further removed. Full text of 55 articles was read. All the references of these 55 articles were checked and two more articles were added from registries. These 57 articles were divided based on the comorbidities as mentioned in the flow chart 1 [Table Fig-1].
Flow Chart of number of studies screened and included.
Prevalence of Comorbidities
The hospitalisation rate due to COVID-19 is 4.9 per 100,000 population. Approximately, 90% of the patients hospitalised have comorbidities according to the Center for Disease Control and prevention (CDC). COVID- NET is a newly created COVID-19 surveillance team in the United State America (USA). According to whom, the prevalence of co-morbidities was at 89.3% in hospitalised patients [8]. The most common and major comorbidities were hypertension, diabetes, obesity, CKD, cardiovascular disease, malignancies and pre-existing lung disorders [8]. Elderly (>65 years) patients were at higher risk of hospitalisation and death. A total of 94.4% of elderly patients had comorbidities. The most common underlying condition in elderly was hypertension with a prevalence of 72% followed by cardiovascular disease, obesity, diabetes and respiratory illness. Among the younger age group of patients, obesity was most common with prevalence of 49% in the age group of 50- to 64 years and 59% in those between 18 to 49 years [8].
Number of Comorbidites
Guan WJ et al., studied 1590 lab confirmed COVID-19 patients in China [9]. Out of 1590 patients, 25% had atleast one comorbidity. The prevalence of hypertension was most common (16%). Other significant co-morbidities were cardiovascular diseases, diabetes, respiratory diseases like Chronic Obstructive Pulmonary Disease (COPD), Chronic Kidney Disease (CKD), malignancy and immunodeficiency syndromes. Two or more co-morbidities were commonly seen in severe cases than in nonsevere cases. These patients were likely to be older, presenting more commonly with shortness of breath followed by nausea or vomiting, unconsciousness and less abnormal chest x-ray compared to patients with one comorbidity. Yang J et al conducted a meta-analysis [10], which included seven chinese observational studies, with a total of 1527 infected patients. On comparing severe and nonsevere cases, pooled odds ratio for hypertension, respiratory illness and cardiovascular disease were 2.36, 2.46 and 3.42, respectively. They concluded that patients with comorbidities may be at risk of severe disease compared to those without.
Age
Liu Y et al., in China studied the association between age and clinical characteristics and outcome of COVID-19 illness [11]. They studied 221 COVID-19 positive patients. These patients were divided into two groups- less than 60 years of age and more than 60 years of age. The primary outcome of the disease course and secondary outcome of respiratory failure was compared between both the groups. Patients in older age group presented with higher severity of illness and higher levels of Blood Urea Nitrogen (BUN), Lactate Dehydrogenase (LDH), lymphopenia compared to those <60 years of age. Older patients had more lobes of lung involvement and secondary bacterial coinfection. The disease course was longer and respiratory failure rates were higher in this group.
Death and hospitalisation rates have been compiled by the CDC which is summarised in [Table/Fig-2] [12]. They have taken age group between 18 to 29 years as the comparison group because they have a strong immunity and compared the rest of the age groups with this particular group [Table/Fig-3] [11,13-18].
Death and hospitalisation rates according to age.
| Age | Hospitalisation rates | Death rates |
|---|
| 0-4 years | Four times lesser | Nine times lesser |
| 5-17 years | Nine times lesser | Sixteen times lesser |
| 8-29 years | Comparison group | Comparison group |
| 30-39 years | Twice higher | Four times higher |
| 40-49 years | Thrice higher | Ten times higher |
| 50-64 years | Four times higher | Thirty times higher |
| 65-74 years | Five times higher | Ninety times higher |
| 75-85 years | Eight times higher | Two hundred and twenty times higher |
| >85 years | Thirteen times higher | Six hundred and thirty times higher |
Association of age and gender with COVID-19 [11,13-18].
| Variables | Supporting studies | Number of patients | Place of study | Outcome |
|---|
| Age | Liu Y et al., [11] | 221 | Shangai, China | Elderly patients suffered more severe illness |
| Chen N et al., [13] | 99 | Wuhan, China |
| Zhang JJ et al., [14] | 140 | Wuhan, China |
| Wang D et al., [15] | 138 | Wuhan, China |
| Petrilli CM et al., [16] | 5279 | New York city, USA |
| Gender | Jin JM et al., [17] | 604 | Wuhan and Beijing, China | Males had higher severity of illness |
| Chen T et al., [18] | 799 | Wuhan china | Males were more prone for severe illness |
Few other case series have also showed that patients older than 65 had severe COVID-19 illness [13-15]. Initial reports showed an increase prevalence of COVID-19 in elderly population [16]. It is important to know that in the elderly, weight and muscle mass decline as age advances, but relative fat mass increases. Elderly are hypertensive and diabetic due to stiffer vessels and impaired metabolic efficiency, respectively. People who are older (>70 years of age), have less cardiorespiratory reserve to cope with COVID-19 infection just like the young obese people. Immune senescence and inflammageing is well known to increase the risk of disease severity in elderly [19].
Gender
A study by Jin JM et al., has showed that men developed more severe illness compared to women irrespective of the age group [17]. Among the deceased patients, men were 2.4 times more in number compared to women. Though males and females were equally susceptible to the disease, males died more than females. The reason for increase in mortality in males in unexplained [Table/Fig-3]. Few theories have been proposed. Males smoke and consume alcohol more than women. There are more Angiotensin Converting Enzyme 2 (ACE-2) receptors in men compared to women. Females have more resistance to infections than men maybe due to hormonal differences. Also, women are considered to be more responsible than men, hence they practice preventive measures such as hand washing, wearing masks, social distancing more carefully than men [20].
Hypertension
Many hypertensive patients are on Angiotensin Converting Enzyme (ACEi) inhibitors and Angiotensin Receptor Blockers (ARBs). These drugs can theoretically increase the levels of ACE-2 receptors [21], which are the entry point for the virus. Hence, increasing the severity of illness. Few other experimental studies have shown ACE-2 to be protective against lung injury. ACE-2 breakdown Angiotensin II to Angiotensin [1-7] which is anti-inflammatory in action. Hence, it reduces the inflammation and prevents damage to major organs [22]. Few other studies have shown that Increased ACE-2 levels bind to SARS-Coronavirus in circulation and in turn reduce the injury to lungs and other major organs like the heart and kidneys [23]. In fact, recombinant ACE-2 is being studied as treatment for COVID-19, it reduces organ damage by binding to circulating virus. Protecting major organs like lungs, heart and kidney. Thus, reducing the chances of acute lung injury, myocarditis, acute kidney injury [23]. More studies are required to assess the risks or benefits of ACEi or ARBs in COVID-19 disease. Current guidelines recommend not to withhold ACEi or ARBs in hypertensive patients with COVID [Table/Fig-4] [24-28].
Studies which demonstrated hypertension as a risk factor for COVID-19 [24-28].
| Supporting studies | Place of study | Number of patients | Outcome |
|---|
| Andrew Ip et al [24] | New Jersy, USA | 1584 with HTN | Mortality increased with HTN |
| Yang G et al., [25] | Wuhan, China | 126 with HTN | ACEi/ARBs reduced death rates |
| Liu Y et al., [26] | Shenzen, China | 46 with HTN | ARB’s reduced severity of illness |
| Zhang L et al., [27] | Wuhan, China | 90 with HTN | Calcium channel blockers reduced mortality rate better than ACEi/ARBs |
| Zeng Z et al., [28] | Wuhan, China | 274 with HTN | ACEi/ARBs increased the severity of illness |
HTN: Hypertension; ACEi: Angiotensin converting enzyme inhibitors; ARBs: Angiotensin receptor blockers
Diabetes
Diabetes is another important risk factor for mortality in COVID-19. As such, mortality rates in people aged above 75 years was higher in diabetics with pneumonia when compared to mortality due to cardiovascular disease or cancer in the same age group [29]. Similar results hold good for the two earlier corona virus infections. SARS which started in 2002 and affected more than 8000 people mainly in Asia [30]. Middle East Respiratory Syndrome (MERS) affected more than 2000 people mainly in Saudi Arabia [31]. Diabetes compromises the innate immune system and creates a pro-inflammatory cytokine milieu. Theoretically, it reduces the expression of ACE-2 and use of ACEi or ARB may contribute to poor prognosis. On the other hand, direct beta cell damage, insulin resistance due to cytokines, hypokalemia and use of drugs such as corticosteroids, lopinavir/ritonavir can worsen the glucose control in patients with diabetes mellitus. The two-way interaction between diabetes and COVID-19 is like a vicious cycle. COVID-19 worsens dysglycaemia and diabetes exacerbates the severity of COVID-19. Hence, it is important to maintain good glycaemic control to prevent COVID-19 illness and its severity in people with diabetes mellitus [Table/Fig-5] [32-35].
Studies which demonstrated diabetes as a risk factor for COVID-19 [32-35].
| Supporting studies | Place of study | Number of patients | Outcome |
|---|
| Zhang Y et al., [32] | Wuhan, China | 258 | Diabetes Mellitus (DM) is associated with greater disease severity and higher mortality |
| Deng SQ et al., [33] | Wuhan, China | 26 | Diabetes associated with more severe disease |
| Bello-Chavolla OY et al., [34] | Mexico | 137 | Diabetes was categorised as mild risk |
| Guo W et al., [35] | Wuhan, China | 174 | Diabetes increased the risk of progression and also is a cause of poor prognosis |
Obesity
Obesity is one of the pre-existing diseases associated with deaths in COVID-19. The increase in number of deaths in Italy and USA compared to China can be attributed to obesity [36]. Furthermore, the increase in prevalence of obesity in USA [37] and prior experience showing increase in mortality in obese patients with H1N1 influenza [38], should sensitise the clinicians to plan for an aggressive treatment for COVID-19 in obese patients. Obesity is associated with decreased functional capacity, expiratory reserve volume, and respiratory system compliance. When patients lie down in supine position, the diaphragm gets pushed up compromising the ventilatory capacity [38]. Also, increased pro-inflammatory cytokines associated with obesity may cause increased morbidity [38]. All these factors emphasise on the need for increased vigilance, priority for detection and testing and more aggressive treatment in this group of patients [Table/Fig-6] [39-42].
Studies which demonstrated obesity as a risk factor for COVID-19 [39-42].
| Supporting studies | Place of study | Number of patients | Outcome |
|---|
| Palaiodimos L et al., [39] | New York, USA | 200 | Severe Obesity was associated with higher in hospital mortality |
| Nakeshbandi M et al., [40] | New York, USA | 139 | Overweight and Obesity is a risk factor for mortality and intubation. |
| Simonnet A et al., [41] | France | 124 | Obesity is a risk factor for increasing severity of COVID-19 |
| Cai SH et al., [42] | China | 96 | Higher BMI was associated with poor prognosis |
Chronic Kidney Disease (CKD)
Patients on chronic dialysis are prone for increased risk of mortality. The severity of infection is high as they have suppressed immune response due to uremia. They are also exposed multiple number of times to the hospital environment and are at higher risk of acquiring infection as well. There is a huge burden on the dialysis facility as they have to ensure the safety of the staff, follow isolation procedures and prevent infection. Home dialysis or peritoneal dialysis is always preferred and tele-consultation can be provided for the same [43]. In a study conducted at a haemodialysis center at Wuhan, China found that the risk of acquiring infection was higher in haemodialysis patients. The centers for haemodialysis can be the source of spread of infection. However, they usually have mild illness and are unlikely to progress to severe illness as they have impaired cell mediated immunity and are less likely to mount a cytokine storm in response to infection. Mortality was mainly due to cardiovascular causes. Hence, the effect of COVID-19 on cardiovascular system needs to be studied [44]. Few studies found that the outcomes of COVID-19 in haemodialysis patients was not poor and attributed it to earlier detection and treatment with antivirals [44,45]. However, other studies have shown poor outcomes in patients with kidney disease [Table/Fig-7] [46-48].
Studies which demonstrated kidney disease as a risk factor for COVID-19 [46-48].
| Supporting studies | Place of study | Number of patients | Outcome |
|---|
| Zhou H et al., [46] | Wuhan, China | 701 | Higher risk of in hospital death |
| Cheng Y et al., [47] | Wuhan, China | 178 | Increase in creatinine and Blood urea nitrogen associated with poor prognosis |
| Alberici F et al., [48] | Italy | 20 | Rapid deterioration clinically in patients with worsening lung radiology findings |
Cardiovascular Disease
A meta-analysis of six studies which included 1527 patients found an increase in morbidity and mortality in patients with underlying metabolic cardiovascular disease and COVID-19. An 8% of patients had acute cardiac injury. Also, cardiac injury was more commonly seen in patients admitted in ICU and in those with severe illness compared to mild illness and non-ICU patients [49]. Patients with underlying ST-Segment Elevation Myocardial Infarction (STEMI) or unstable angina have poor cardiac reserve and more susceptibility to infection. According to Shanghai health commission, the first COVID death was an 88-year-old with pre-existing hypertension and cardiac dysfunction. The patient died of cardiac failure and multi-organ dysfuction. Autopsy study showed that, COVID-19 infection precipitated the causes of death [50]. Many mechanisms of injury are proposed. First of all, the virus can cause direct cardiac myocyte injury as studied by Oudit GY et al., in Toronto, Canada during SARS outbreak. When mice were infected with SARS-Cov, it caused pneumonia and ACE-2 dependent cardiac infection [50]. Secondly, hypoxia causes myocardial injury. Pneumonia causes reduced gas exchange in the lungs leading to hypoxemia which in turn hampers cell metabolism. Anaerobic fermentation caused release of free radicals and lactic acidosis. Free radicals injury the phospholipid cell membranes causing cell apoptosis and organ damage [51]. Thirdly, patients with high inflammatory markers caused be cytokine storm had more severe illness [51]. Also, other factors such as anxiety and certain medications caused repeated increase in catecholamine levels causing myocardial cell damage [Table/Fig-8] [13,15,52, 53].
Studies which demonstrated cardivascular disease as a risk factor for COVID-19 [13,15,52, 53].
| Supporting studies | Place of study | Number of patients | Outcome |
|---|
| Chen N et al [13] | Wuhan, China | 40 out of 99 had CVD | Underlying cardiovascular disease significantly increased the mortality risk |
| Wang D et al., [15] | Wuhan, China | 20 out of 138 had CVD |
| Guan WJ et al., [52] | China | 27 out of 1099 had CVD |
| Wu J et al., [53] | China | 25 out of 80 |
CVD: Cardiovascular disease
Lung Disorders
COPD is associated with increased risk of morbidity and mortality in COVID-19 infection [54]. Many mechanisms contribute to increased risk such as impaired host immunity, imbalance in microbiome, persistent mucus production, structural injury of the lung, altered local and systemic inflammatory response and use of steroid inhalation. Some studies have showed an increased level of ACE-2 in COPD patients with COVID-19. However, more studies are required to prove it [54,55].
Malignancy
The studies mentioned below showed that the cancer patients had higher severity of illness, higher risk of intubation and higher mortality rates compared to the general population. Most commonly elder patients (>65 years) were affected with malignancy. Patients who underwent chemotherapy or onco-surgery during the previous month were particularly at higher risk. Also, they deteriorated rapidly compared to the general population [56,57].
Hence, few strategies are proposed to protect these groups of patients. Firstly, any chemotherapy and onco-surgery should be postponed in stable malignancy patients. Secondly, they should wear personal protective equipment at all times. Thirdly, they should be treated for COVID-19 aggressively especially elderly patients [Table/Fig-9] [56-58].
Studies which demonstrated malignancy as a risk factor for COVID-19 [56-58].
| Supporting studies | Place of study | Number of patients | Outcome |
|---|
| Liang W et al., [56] | China | 12 | Cancer patients were at higher risk of COVID-19 |
| Zhang L et al., [57] | Wuhan, China | 28 | Cancer patients showed poor outcomes |
| Yu J et al., [58] | Wuhan, China | 18 | Intensive treatment and surveillance is required for cancer patients |
A study was conducted in Beijing, China where they included all severely ill patients admitted in critical care ward. There were 69 patients included in the study. These patients were divided into three categories- Category A: Patients with only pneumonia, Category B: Patients with pneumonia and pre-existing comorbidities, Category C: Patients with severe illness due to worsening of either Category A or B.
Type A patients received basic treatment like steroids, antivirals, antibiotics and oxygen therapy. In Type B patients, they monitored the trend of the status of comorbidities while treating for pneumonia. Type C patients included those in type A and type B who deteriorated with multi-organ dysfunction. 22% in Group A, 55% in group B and 23% in Group C were present. They concluded that importance should be given to the organ function and supportive treatment in the form of ventilation and renal replacement therapy. Therapy should be individualised based on the organ involved. Aggravation of the underlying comorbid condition due to COVID was the main cause of death. Hence, it is important to emphasise on the treatment of underlying comorbidities while treating pneumonia. COVID-19 causes not only pneumonia but also multi-organ injury like cardiac arrythmias, acute myocardial injury, acute renal injury, acute hepatic dysfunction, coagulation abnormalities, lymphopenia, neutrophilia and so on. Patient may ultimately succumb to multi organ injuries [59].
Conclusion(s)
Overall, circulatory and endocrine diseases were found to be the most common pre-existing condition in the form of hypertension and diabetes mellites, respectively. It should be noted that the observed frequency of comorbidity may be confounded by the transmission dynamics within particular age groups, case detection or testing protocols or hospital admission policies during the earlier phase of the epidemic. However, the percentage of COVID-19 patients with renal disease and malignancy were low. Immune dysregulation and prolonged inflammation caused by COVID-19 infection in patients with comorbidities are the key causes for increased risk of death in this population.
It is also well known that some of the comorbidities co-exist. For example, diabetes and COPD are frequently seen in patients with hypertension or cardiac disease. Hence, people with comorbidities have prior poor baseline health characteristics and are prone for more severe disease. Furthermore, as the number of comorbidities increased, the severity of the disease also proportionally increased. A meticulous triage of patients should be carried out after acquiring proper medical history because this will help to identify patients who are at an increased risk of poor outcome of the infection. Also, they should be given more aggressive treatment upon diagnosis of infection.
[1]. Hui DS, Ia E, Madani TA, Ntoumi F, Kock R, Dar O, The continuing 2019- nCoV epidemic threat of novel coronaviruses to global health- the latest 2019 novel coronavirus outbreak in Wuhan, ChinaInt J Infect Dis 2020 19:264-66.10.1016/j.ijid.2020.01.00931953166 [Google Scholar] [CrossRef] [PubMed]
[2]. Cucinotta D, Vanelli M, WHO Declares COVID-19 a PandemicActa Biomed 2020 91(1):157-60. [Google Scholar]
[3]. Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): A population-based studyLancet 2020 396(10247):313-19.10.1016/S0140-6736(20)31304-0 [Google Scholar] [CrossRef]
[4]. John Hopkins University Covid-19 dashboard. Available from: https://coronavirus.jhu.edu/map.html.(Accessed on July 06, 2020) [Google Scholar]
[5]. Centers for Disease Control and Prevention. Commercial Laboratory Seroprevalence Survey Data. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/commercial-lab-surveys.html (Accessed on July 06, 2020) [Google Scholar]
[6]. Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ, Seroprevalence of antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020JAMA Intern MedPublished online July 21, 202010.1001/jamainternmed.2020.413032692365 [Google Scholar] [CrossRef] [PubMed]
[7]. Aromataris E, Munn Z, JBI Manual for Evidence SynthesisJBI 2020 Available from https://doi.org/10.46658/JBIMES-20-0210.46658/JBIMES-20-02 [Google Scholar] [CrossRef]
[8]. Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, Hospitalisation Rates and characteristics of patients hospitalised with Laboratory- Confirmed Coronavirus Disease 2019- COVID-NET, 14 States, March 1-30, 2020MMWR Morb Mortal Wkly Rep 2020 69:458-64.10.15585/mmwr.mm6915e3 [Google Scholar] [CrossRef]
[9]. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, LI Y-M, Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysisEur Respir J 2020 55:200054710.1183/13993003.00547-202032217650 [Google Scholar] [CrossRef] [PubMed]
[10]. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysisInt J Infect Dis 2020 94:91-95.10.1016/j.ijid.2020.03.01732173574 [Google Scholar] [CrossRef] [PubMed]
[11]. Liu Y, Mao B, Liang S, Yang JW, Lu HW, Chai YH, Shanghai Clinical Treatment Experts Group for COVID-19. Association between age and clinical characteristics and outcomes of COVID-19Eur Respir J 2020 55(5):200111210.1183/13993003.01112-202032312864 [Google Scholar] [CrossRef] [PubMed]
[12]. Covid 19 hospitalisation and death rate by age. CDC, USA. [Updated on August 18, 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalisation-death-by-age.html. (accessed on 20/09/2020) [Google Scholar]
[13]. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive studyLancet 2020 395:507-13.10.1016/S0140-6736(20)30211-7 [Google Scholar] [CrossRef]
[14]. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, ChinaAllergy 2020 75(7):1730-41.10.1111/all.1423832077115 [Google Scholar] [CrossRef] [PubMed]
[15]. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Clinical characteristics of 138 hospitalised patients with 2019 novel coronavirus-infected pneumonia in Wuhan, ChinaJAMA 2020 323(11):1061-69.10.1001/jama.2020.158532031570 [Google Scholar] [CrossRef] [PubMed]
[16]. Petrilli CM, Jones A, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort studyBMJ 2020 369:m196610.1136/bmj.m196632444366 [Google Scholar] [CrossRef] [PubMed]
[17]. Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, Gender differences in patients with COVID-19: Focus on severity and mortalityFront Public Health 2020 29(8):15210.3389/fpubh.2020.0015232411652 [Google Scholar] [CrossRef] [PubMed]
[18]. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective studyBMJ 2020 26(368):m109110.1136/bmj.m109132217556 [Google Scholar] [CrossRef] [PubMed]
[19]. Sattar N, McInnes IB, McMurray JJV, Obesity is a risk factor for severe COVID-19 infection: Multiple potential mechanismsCirculation 2020 142(1):04-06.10.1161/CIRCULATIONAHA.120.04765932320270 [Google Scholar] [CrossRef] [PubMed]
[20]. Bwire GM, Coronavirus: Why men are more vulnerable to Covid-19 than women?SN Compr Clin Med 2020 4:01-03.10.1007/s42399-020-00341-w32838138 [Google Scholar] [CrossRef] [PubMed]
[21]. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2Circulation 2005 111(20):2605-10.10.1161/CIRCULATIONAHA.104.51046115897343 [Google Scholar] [CrossRef] [PubMed]
[22]. Phadke M, Saunik S, Rapid response: Use of angiotensin receptor blockers such as Telmisartan, Losartsan in nCoV Wuhan Corona Virus infections-novel mode of treatment. Response to the emerging novel coronavirus outbreakBr Med J 2020 368:m40610.1136/bmj.m40632005675 [Google Scholar] [CrossRef] [PubMed]
[23]. Batlle D, Wysocki J, Satchell K, Soluble angiotensin-converting enzyme 2: A potential approach for coronavirus infection therapy?Clin Sci (Lond) 2020 134:543-45.10.1042/CS2020016332167153 [Google Scholar] [CrossRef] [PubMed]
[24]. Andrew IP, Parikh K, Parrillo JE, Mathura S, Hansen E, Sawczuk IS, Hypertension and renin angiotensin aldosterone system inhibitors in patients with covid-19medRxiv 2020.04.24.20077388 [Preprint] 2020 [cited 2020 Aug 08] Available from: https://www.medrxiv.org/content/10.1101/2020.04.24.20077388v1 [Google Scholar]
[25]. Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, Effects of Angiotensin II receptor blockers and ACE (Angiotensin-Converting Enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID-19 and Hypertension: A Single-Center Retrospective studyHypertension 2020 76(1):51-58.10.1161/HYPERTENSIONAHA.120.1514332348166 [Google Scholar] [CrossRef] [PubMed]
[26]. Liu Y, Huang F, Xu J, Yang P, Qin Y, Cao M, Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patientsmedRxiv 2020.03.20.20039586 [Preprint] 2020 [cited 2020 Aug 08] Available from https://www.medrxiv.org/content/10.1101/2020.03.20.20039586v110.1101/2020.03.20.20039586 [Google Scholar] [CrossRef]
[27]. Zhang L, Sun Y, Zeng H, Peng Y, Jiang X, Shang WJ, Calcium channel blocker amlodipine besylate is associated with reduced case fatality rate of COVID-19 patients with hypertensionmedRxiv 2020.04.08.20047134 [Preprint] 2020 [cited 2020 Aug 08] Available from https://www.medrxiv.org/content/10.1101/2020.04.08.20047134v110.1101/2020.04.08.20047134 [Google Scholar] [CrossRef]
[28]. Zeng Z, Sha T, Zhang Y, Wu F, Hu H, Li H, Hypertension in patients hospitalised with COVID-19 in Wuhan, China: A single-center retrospective observational studymedRxiv.2020.04.06.20054825 [Preprint] 2020 Available from https://www.medrxiv.org/content/10.1101/2020.04.06.20054825v110.1101/2020.04.06.20054825 [Google Scholar] [CrossRef]
[29]. Wu H, Lau ESH, Ma RCW, Kong APS, Wild SH, Goggins W, Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001-2016: A retrospective cohort studyDiabetologia 2020 63(4):757-66.10.1007/s00125-019-05074-731942668 [Google Scholar] [CrossRef] [PubMed]
[30]. Chan-Yeung M, Xu RH, SARS: EpidemiologyRespirology 2003 8:S9-S14.10.1046/j.1440-1843.2003.00518.x15018127 [Google Scholar] [CrossRef] [PubMed]
[31]. Morra ME, Van Thanh L, Kamel MG, Ghazy AA, Altibi AMA, Dat LM, Clinical outcomes of current medical approaches for Middle East respiratory syndrome: A systematic review and meta-analysisRev Med Virol 2018 28:e197710.1002/rmv.197729664167 [Google Scholar] [CrossRef] [PubMed]
[32]. Zhang Y, Cui Y, Shen M, Zhang J, Liu B, Comorbid diabetes mellitus was associated with poorer prognosis in patients with COVID-19: A retrospective cohort studymedRxiv 2020 10.1101/2020.03.24.20042358 [Google Scholar] [CrossRef]
[33]. Deng SQ, Peng HJ, Characteristics of and public health responses to the coronavirus disease 2019 outbreak in ChinaJ Clin Med 2020 2(E575):910.3390/jcm902057532093211 [Google Scholar] [CrossRef] [PubMed]
[34]. Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, Vargas-Vázquez A, González-Díaz A, Márquez-Salinas A, Predicting mortality due to SARS-CoV-2: A mechanistic score 2 relating obesity and diabetes to COVID-19 outcomes in MexicoThe Journal of Clinical Endocrinology & Metabolism 2020 105(8):2752-61.medRxiv; 202010.1210/clinem/dgaa34632474598 [Google Scholar] [CrossRef] [PubMed]
[35]. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Diabetes is a risk factor for the progression and prognosis of COVID-19Diabetes Metabolism Research and Reviews 2020 31:e331910.1002/dmrr.33197228407 [Google Scholar] [CrossRef] [PubMed]
[36]. Onder G, Rezza G, Brusaferro S, Case-fatality rate and characteristics of patients dying in relation to COVID-19 in ItalyJAMA 2020 323(18):1775-76.10.1001/jama.2020.468332203977 [Google Scholar] [CrossRef] [PubMed]
[37]. Hales K, Carroll MD, Fryar CD, Ogden CL, Prevalence of obesity and severe obesity among adults: United States, 2017-2018NCHS Data Brief, no. 360 2020 Hyattsville, MDNational Center for Health Statistics10.4065/mcp.2010.016620664021 [Google Scholar] [CrossRef] [PubMed]
[38]. Venkata C, Sampathkumar P, Afessa B, Hospitalised patients with 2009 H1N1 influenza infection: The Mayo Clinic experienceMayo Clin Proc 2010 85:798-805. [Google Scholar]
[39]. Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New YorkMetabolism 2020 108:15426210.1016/j.metabol.2020.15426232422233 [Google Scholar] [CrossRef] [PubMed]
[40]. Nakeshbandi M, Maini R, Daniel P, Rosengarten S, Parmar P, Wilson C, The impact of obesity on COVID-19 complications: A retrospective cohort studyInt J Obes (Lond) 2020 44(9):1832-37.10.1038/s41366-020-0648-x32712623 [Google Scholar] [CrossRef] [PubMed]
[41]. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, LICORN and the Lille COVID-19 and obesity study groupHigh prevalence of obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilationObesity (Silver Spring) 2020 28(7):1195-99.10.1002/oby.2283132271993 [Google Scholar] [CrossRef] [PubMed]
[42]. Cai SH, Liao W, Chen SW, Liu LL, Liu SY, Zheng ZD, Association between obesity and clinical prognosis in patients infected with SARS-CoV-2Infect Dis Poverty 2020 9(1):8010.1186/s40249-020-00703-532600411 [Google Scholar] [CrossRef] [PubMed]
[43]. Basile C, Combe C, Pizzarelli F, Covic A, Davenport A, Kanbay M, Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centresNephrol Dial Transplant 2020 35(5):737-41.10.1093/ndt/gfaa06932196116 [Google Scholar] [CrossRef] [PubMed]
[44]. Ma Y, Diao B, Lv X, Zhu J, Liang W, Liu L, COVID-19 in hemodialysis (HD) patients: Report from one HD center in Wuhan, ChinamedRxiv 2020 10.1101/2020.02.24.20027201 [Google Scholar] [CrossRef]
[45]. Jung HY, Lim JH, Kang SH, Kim SG, Lee YH, Lee J, Outcomes of COVID-19 among Patients on In-Center Hemodialysis: An Experience from the Epicenter in South KoreaJ Clin Med 2020 9(6):E168810.3390/jcm906168832498262 [Google Scholar] [CrossRef] [PubMed]
[46]. Zhou H, Zhang Z, Fan H, Li J, Li M, Dong Y, Urinalysis, but not blood biochemistry, detects the early renal-impairment in patients with COVID-19MedRxiv 2020 10.1101/2020.04.03.20051722 [Google Scholar] [CrossRef]
[47]. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Kidney disease is associated with in-hospital death of patients with COVID-19Kidney Int 2020 97:829e3810.1016/j.kint.2020.03.00532247631 [Google Scholar] [CrossRef] [PubMed]
[48]. Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumoniaKidney Int 2020 97(6):1083e810.1016/j.kint.2020.04.00232354634 [Google Scholar] [CrossRef] [PubMed]
[49]. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in ChinaClin Res Cardiol 2020 109(5):531-38.10.1007/s00392-020-01626-932161990 [Google Scholar] [CrossRef] [PubMed]
[50]. Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, SARS- coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARSEur J Clin Invest 2009 39:618-25.10.1111/j.1365-2362.2009.02153.x19453650 [Google Scholar] [CrossRef] [PubMed]
[51]. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, ChinaThe Lancet 2020 395(10223):497-506.10.1016/S0140-6736(20)30183-5 [Google Scholar] [CrossRef]
[52]. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Clinical characteristics of coronavirus disease 2019 in ChinaN Engl J Med 2020 382:1708-20.10.1056/NEJMoa20020327092819 [Google Scholar] [CrossRef] [PubMed]
[53]. Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, Clinical Characteristics of imported cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu province: A multicenter descriptive studyClin Infect Dis 2020 71(15):706-12.10.1093/cid/ciaa19932109279 [Google Scholar] [CrossRef] [PubMed]
[54]. Wan Y, Shang J, Graham R, Baric RS, Li F, Receptor recognition by the novel coronavirus from wuhan: An analysis based on decade-long structural studies of SARS CoronavirusJ Virol 2020 94(7):e00127-20.10.1128/JVI.00127-20 [Google Scholar] [CrossRef]
[55]. Ayada C, Toru Ü, Genç O, Şahin S, Turgut S, Turgut G, Angiotensinogen gene M235T and angiotensin II-type 1 receptor gene A/C1166 polymorphisms in chronic obtructive pulmonary diseaseInt J Clin Exp Med 2015 8(3):4521-26. [Google Scholar]
[56]. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Cancer patients in SARS CoV-infection: A nationwide analysis in ChinaLancet Oncol 2020 21(3):335e710.1016/S1470-2045(20)30096-6 [Google Scholar] [CrossRef]
[57]. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, ChinaAnn Oncol 2020 31(7):894-e901.10.1016/j.annonc.2020.03.29632224151 [Google Scholar] [CrossRef] [PubMed]
[58]. Yu J, Ouyang W, Chua MLK, Xie C, SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in wuhan, ChinaJAMA Oncology 2020 2020:e20098010.1101/2020.02.22.20025320 [Google Scholar] [CrossRef]
[59]. Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Comorbidities and multi-organ injuries in the treatment of COVID-19Lancet 2020 395(10228):e5210.1016/S0140-6736(20)30558-4 [Google Scholar] [CrossRef]