Point Mutations in Muscle Segment Homeobox 1 (MSX1) Gene in an Individual with Mandibular Retrognathia: A Case Report
Dechamma Pandyanda Nanjappa1, Murali Patla Shivarama Bhat2, Veena Shetty3, Krishna Nayak Uppinagadi Shroof4, Anirban Chakraborty5
1 Research Scholar, Division of Molecular Genetics and Cancer, Nitte University Centre for Science Education and Research (NUCSER), Nitte (Deemed to be University), Deralakatte, Mangaluru, Karnataka, India.
2 Assistant Professor, Department of Orthodontics and Dentofacial Orthopaedic, AB Shetty Memorial Institute of Dental Sciences, Nitte (Deemed to be University), Deralakatte, Mangaluru, Karnataka, India.
3 Professor, Department of Microbiology, KS Hedge Medical Academy, Nitte (Deemed to be University), Deralakatte, Mangaluru, Karnataka, India.
4 Professor, Department of Orthodontics, AB Shetty Memorial Institute of Dental Sciences, Nitte (Deemed to be University), Deralakatte, Mangaluru, Karnataka, India.
5 Professor, Division of Molecular Genetics and Cancer, Nitte University Centre for Science Education and Research (NUCSER), Nitte (Deemed to be University), Deralakatte, Mangaluru, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Anirban Chakraborty, Division of Molecular Genetics and Cancer, Nitte University Centre for Science Education and Research (NUCSER), Paneer Campus, Kotekar-Beeri Road, Nitte (Deemed to be University), Deralakatte, Mangaluru, Karnataka, India.
E-mail: anirban@nitte.edu.in
Malocclusion is an orofacial anomaly that manifests in the form of misaligned dental arches. Mandibular retrognathia is a type of malocclusion, characterised by defective mandibular bone growth. Muscle Segment Homeobox (MSX) gene family, plays an essential role during embryonic development by coordinating processes that decide the patterning and morphogenesis of tissues. Expression of MSX1 and MSX2 genes in the maxilla, mandible and the mesenchymal cells of cephalic neural crest strongly suggest their role in craniofacial development. Here, point mutations (T8I, P11S and A68V) in the coding region of MSX1 gene in a 20-year-old male patient with severe mandibular retrognathia was reported. To date, there has been no report on the association of MSX genes with mandibular anomalies. Evaluating, the significance of these novel mutations through functional studies in animal models will lead to a better understanding of the role of MSX genes in mandibular morphogenesis.
Craniofacial development, Malocclusion, Misaligned dental arches
Case Report
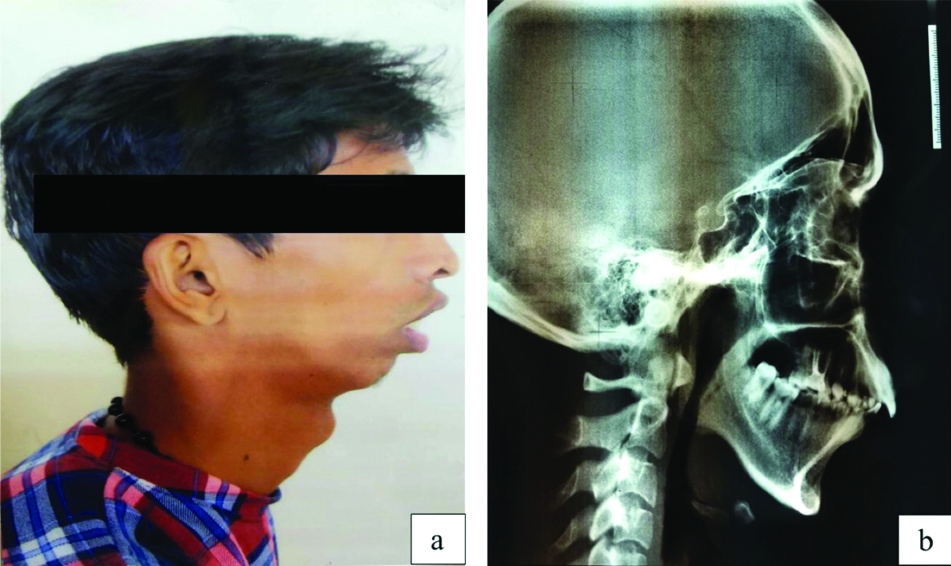
A 20-year-old male (referred to as subject A) from the northern parts of Kerala, India reported at the Orthodontics Department of Mangalore, India with issues related to forwardly placed front teeth, facial appearance and smile. Upon examination, the radiograph of subject A revealed skeletal class II malocclusion with convex facial profile, retrognathic mandible, orthognathic maxilla, incompetent lip, deep mentolabial sulcus, class II molar and canine relationship. The phenotypic analysis of subject A showed characteristic features of Convex facial profile with Orthognathic Maxilla (SNA=83°) and Retrognathic Mandible (SNB=74°) [Table/Fig-1]. The extraoral image of subject A showed Angle’s class II malocclusion with increased overjet [Table/Fig-2a]. Lateral cephalogram radiograph tracing showed class II skeletal base with retrognathic mandible, average growth pattern, protruded and proclined upper and lower incisors [Table/Fig-2b]. The controls included (Subject AF, subject AM and subject B) did not display clinical features of retrognathia and showed a straight facial profile with Orthognathic Maxilla (SNA=82°) and Mandible (SNB=79°). Extaorally, these subjects had Angle’s Normal occlusion with ideal overjet and overbite.
Details of occlusal analysis and measurements of maxillary and mandibular body of case and control.
| Subject | Maxillary bone position and size | Mandibular bone position and size | Ramus vertical height | Gonial angle | Occlusal analysis |
|---|
| SNA | ANS-PNS | SNB | Co-Gn | Go-Pg | Xi-Pg | Ar-Go | Cf-Go | Ar-Go-Gn | Molar relation-ship | Over-jet |
|---|
| Subject A | 83° | 58 mm | 74° | 101 mm | 67 mm | 62 mm | 42 mm | 58 mm | 129° | Class II | 12 mm |
| Subject B | 81° | 57 mm | 79° | 112 mm | 74 mm | 75 mm | 47 mm | 65 mm | 130° | Class I | 2 mm |
SNA: Sella nasion A point; SNB: Sella nasion B point; ANS: Anterior nasal spine; PNS: Posterior nasal spine; Co: Condyle; Gn: Gnathion; Go: Gonion; Pg: Pogonion; Ar: Articulare; Xi: Xi point; Cf: The point of intersection of the pterygoid root vertical to the Frankfort horizontal plane
Patient phenotype. a) A photograph of subject A showing convex facial profile with Orthognathic Maxilla and Retrognathic Mandible; b) A radiograph of subject A showing class II malocclusion with increased overjet.
Treatment of patients with skeletal Class II malocclusion with retrognathic mandible primarily depends on the severity of skeletal discrepancy and the growth potential of the patient. Growth modulation by means of functional appliance is an ideal treatment for growing individuals whereas, for the patients who have completed the peak growth potential, camouflage and orthognathic surgery are the only available treatment for the comprehensive management. Considering the clinical and radiographic features and the chief complaint of subject A, surgical mandibular advancement with pre and postsurgical orthodontics was recommended. The innumerable factors involved in craniofacial morphogenesis, are the members belonging to MSX gene family. The MSX gene family in mammals include, MSX1, MSX2, and MSX3. While MSX3 is expressed only in the dorsal neural tube, MSX1 and MSX2 are strongly expressed in the regions where epithelial-mesenchymal interaction takes place, particularly in the craniofacial regions [1]. Therefore, the germline status of MSX1 gene was checked in the patient to determine genetic predisposition to the retrognathia. For genetic analysis, peripheral blood was collected from the subject A and from the parents of subject A (father referred to as subject AF and mother referred to as subject AM). In addition, an age-matched control sample with normal mandibular bone growth (referred to as subject B) was also included. Blood genomic DNA was isolated using commercial kit (Macherey Nagel, Germany) following the manufacturer’s instructions. The entire coding region of MSX1 gene was amplified using specific primers and the amplified products were subjected to capillary sequencing (Sanger method). The samples were collected with informed consents and the work was approved by the Central Ethics Committee of Nitte (Deemed to be University).
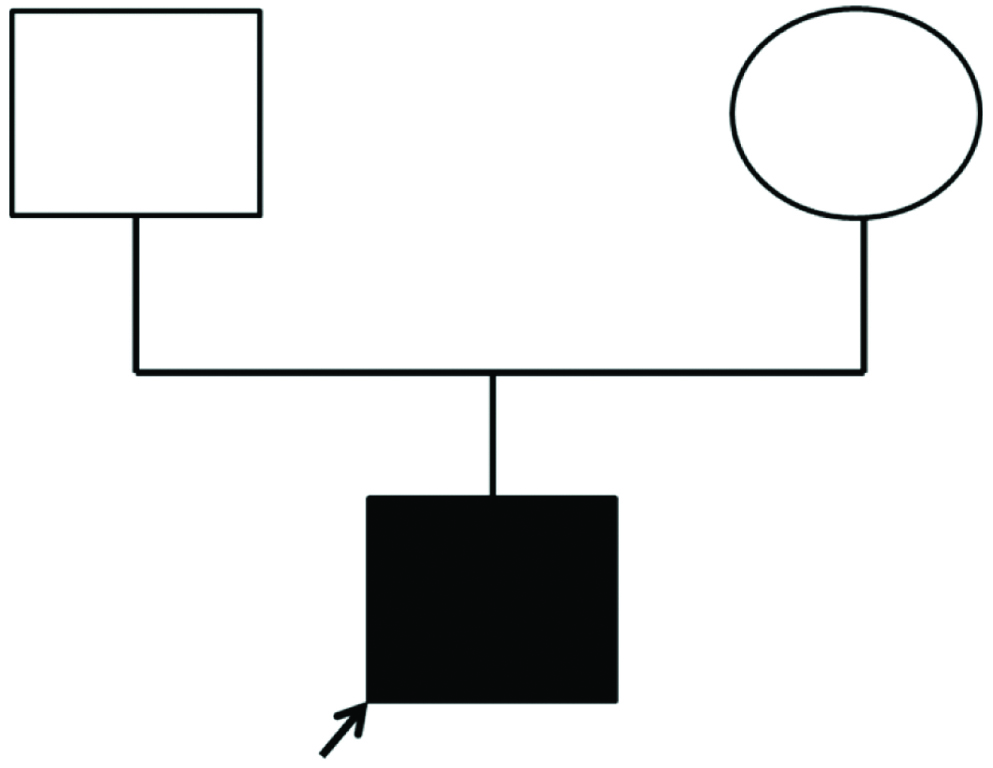
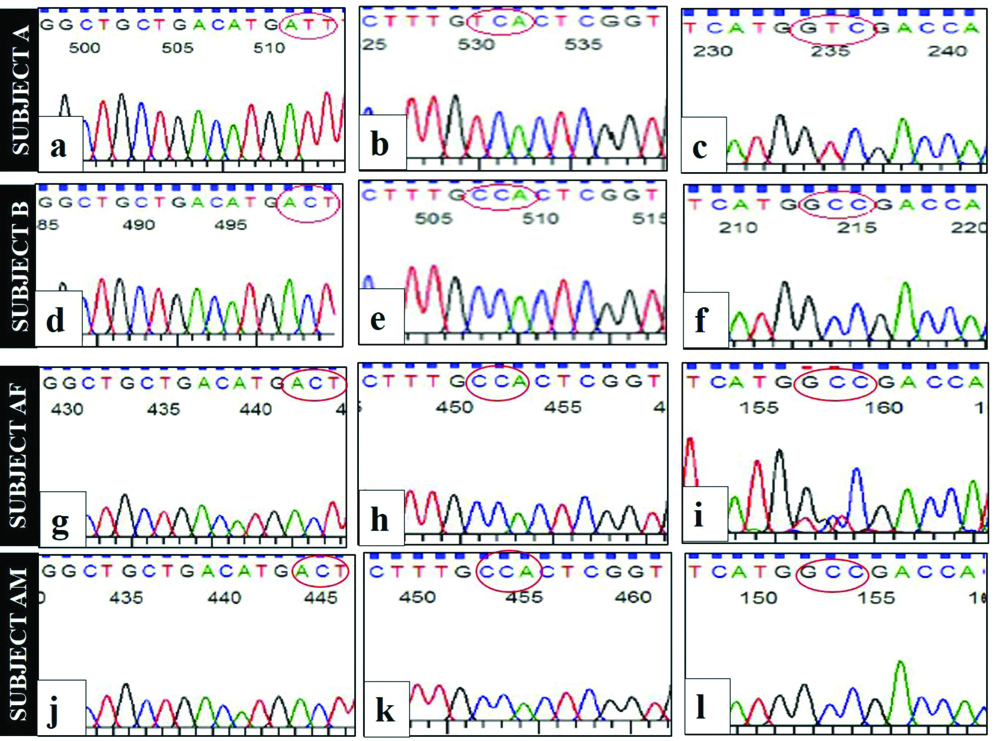
Sanger sequencing of MSX1 gene in subject A showed the presence of point mutations in three locations namely g.4861649C>T (T8I), g.4861657C>T (P11S) and g.4861829C>T (A68V) that resulted in amino acid substitution at codon 8 (T8I), codon 11 (P11S) and codon 68 (A68V). Among these three mutations, T8I and P11S were de novo substitutions, whereas A68V was inherited from the father (subject AF heterozygous for g.4861829C>T) [Table/Fig-3a,b].
A familial pedigree of the proband (subject A).
Sequence analysis of MSX1 gene of proband, the family members and unrelated control.
Electropherograms showing point mutations in subject A at g.4861649C>T (a), g.4861657C>T (b) and g.4861829C>T(c). The highlighted region in red circle represents the affected codons. Subject B, subject AF and subject AM have electropherograms as (d, e, f); (g, h, i) and (j, k, l). Electropherograms of subject B (d,e), subject AF (g,h) and subject AM (j,k) showed wildtype sequence at locations g.4861649 and g.4861657 respectively. While subject AF showed the presence of heterozygous mutation (peaks for both C and T) at g4861829 (i), subject B (f) and subject AM (l) showed wild type sequence at the corresponding site, indicating a carrier state in subject AF. The codes Subject AF and subject AM denote the father and the mother of subject A respectively. Subject B is the age-matched unrelated control with normal mandible
Discussion
Malocclusion is the manifestation of oral-facial anomalies generally occurring due to complex genetic and environmental interactions [2]. Among the various degrees of malocclusions, the frequency of micrognathia is one in 1500 births [3]. Mandibular micrognathia is characterised by defective mandibular bone growth, breathing disorder, sleep apnoea and interference during mastication [4].
Several reports suggest a probable role of MSX genes in human craniofacial development, the most conspicuous being in tooth agenesis and cleft palate [5]. They are also found to be expressed throughout the embryonic stages including the rostral region of the head and mesenchymal cells surrounding the hyomandibular cleft suggesting their involvement in craniofacial development [6,7]. Thus, inductive interactions of the MSX genes are essential for normal craniofacial and ectodermal organ morphogenesis. On the other hand, evidences from literature have implicated Bone Morphogenetic Protein 4 (BMP4) in the development of the medial mandibular region. Deficiency of BMP4 in the mandibular epithelium results in severely shortened mandible [8,9]. Interestingly, there exists a positive feedback loop between BMP4 and MSX1 that regulates the level of both genes in the dental mesenchyme [10]. Although the role of MSX genes in tooth and palate development is undisputed, there has been no report on its role in the development of mandible.
Understanding the aetiology of malocclusion is important, so that orthodontic treatments can focus more on the prevention of these conditions and their underlying skeletal dysplasia. In this case, subject A had skeletal class II malocclusion with convex facial profile, retrognathic mandible, orthognathic maxilla, incompetent lip, deep mentolabial sulcus, class II molar and canine relationship and increased overjet whereas, the parents (subject AF, subject AM) and control (subject B) had orthognathic maxilla and mandible with normal occlusal phenotypic feature. In class II malocclusion, the mandible is significantly protruded than in class I patients with smaller mandibular body and reduced over all mandibular length, similar to what was observed in subject A. Studies related to class II malocclusion show a higher correlation between the patients and immediate family, supporting the concept of the heritability of malocclusion [11,12]. Thus, it is clear that genotype contribute to phenotypic variations. Several genes have been implicated in the development of malocclusion [13]. While most of the studies have focused in class III malocclusions genetics of class I and class II malocclusions are relatively rare [13]. Single nucleotide polymorphisms in PAX9 and NOG genes have been reported in patients with mandibular hypoplasia [9,14].
Conclusion(s)
To date, there has been no report on the role of MSX1 gene in mandible development. The unexpected discovery of mutations in MSX1 gene in a patient with mandibular retrognathia warrants the need for further understanding of the role of MSX genes in mandibular morphogenesis. Future studies must be directed towards screening more patients for these mutations and conducting functional studies in animal models to understand the clinical significance of these mutations.
Funding: This work was supported by a Nitte University a faculty start up research grant to M.P.S.B (Grant number NUFR1/2016/02-04).
Author contribution: DPN and MPSB acquired the clinical data, conducted the laboratory procedures, did literature review, and prepared the first draft. VS and KNUS participated in discussion and assisted in clinical diagnosis. AC and MPSB conceptualised the study. AC designed the study, analysed the results and prepared and edited the final version of the manuscript. All authors reviewed the manuscript and approved the final version. Both DPN and MPSB have the same contribution and equal credit should be given for the same.
SNA: Sella nasion A point; SNB: Sella nasion B point; ANS: Anterior nasal spine; PNS: Posterior nasal spine; Co: Condyle; Gn: Gnathion; Go: Gonion; Pg: Pogonion; Ar: Articulare; Xi: Xi point; Cf: The point of intersection of the pterygoid root vertical to the Frankfort horizontal plane
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 09, 2020
Manual Googling: Nov 05, 2020
iThenticate Software: Dec 12, 2020 (18%)
[1]. Alappat S, Zhang ZY, Chen YP, Msx homeobox gene family and craniofacial developmentCell Res 2003 13(6):42910.1038/sj.cr.729018514728799 [Google Scholar] [CrossRef] [PubMed]
[2]. Sarig R, Slon V, Abbas J, May H, Shpack N, Vardimon AD, Malocclusion in early anatomically modern human: A reflection on the aetiology of modern 123 dental misalignmentPloS one 2013 8(11):e8077110.1371/journal.pone.008077124278319 [Google Scholar] [CrossRef] [PubMed]
[3]. Joshi N, Hamdan AM, Fakhouri WD, Skeletal malocclusion: A developmental disorder with a life-long morbidityJ Clin Med Res 2014 6(6):39910.14740/jocmr1905w25247012 [Google Scholar] [CrossRef] [PubMed]
[4]. Baskaran M, Arularasan SG, Divakar TK, Thirunavukkarasu R, Treatment of micrognathia by intraoral distraction osteogenesis: A prospective studyAnn Maxillofac Surg 2017 7(1):37 [Google Scholar]
[5]. Jumlongras D, Bei M, Stimson JM, Wang WF, DePalma SR, Seidman CE, A nonsense mutation in MSX1 causes Witkop syndromeAm J Hum Genet 2001 69(1):67-74.10.1086/32127111369996 [Google Scholar] [CrossRef] [PubMed]
[6]. Davidson D, The function and evolution of Msx genes: Pointers and paradoxesTrends Genet 1995 11(10):405-11.10.1016/S0168-9525(00)89124-6 [Google Scholar] [CrossRef]
[7]. Han J, Ishii M, Bringas Jr P, Maas RL, Maxson Jr RE, Chai Y, Concerted action of Msx1 and Msx2 in regulating cranial neural crest cell differentiation during frontal bone developmentMech Dev 2007 124(9-10):729-45.10.1016/j.mod.2007.06.00617693062 [Google Scholar] [CrossRef] [PubMed]
[8]. Liu Y, Helms AW, Johnson JE, Distinct activities of Msx1 and Msx3 in dorsal neural tube developmentDevelopment 2004 131(5):1017-28.10.1242/dev.0099414973289 [Google Scholar] [CrossRef] [PubMed]
[9]. Gutiérrez SJ, Gómez M, Rey JA, Ochoa M, Gutiérrez SM, Prieto JC, Polymorphisms of the noggin gene and mandibular micrognathia: A first approximationActa Odontol Latinoam 2010 23(1):13-19. [Google Scholar]
[10]. Tucker AS, Khamis AA, Sharpe PT, Interactions between Bmp4 and Msx1 act to restrict gene expression to odontogenic mesenchymeDev Dyn 1998 212(4):533-39.10.1002/(SICI)1097-0177(199808)212:4<533::AID-AJA6>3.0.CO;2-I [Google Scholar] [CrossRef]
[11]. Bishara SE, Class II malocculsions: Diagnostic and clinical considerations with and without treatmentSem Orthodont 2006 12(1):11-24.10.1053/j.sodo.2005.10.005 [Google Scholar] [CrossRef]
[12]. Mossey PA, The heritability of malocclusion: Part 2. The influence of genetics in malocclusionBrit J Orthodont 1999 26(3):195-203.10.1093/ortho/26.3.19510532158 [Google Scholar] [CrossRef] [PubMed]
[13]. Uribe LMM, Miller SF, Genetics of the dentofacial variation in human malocclusionOrthod Craniofac Res 2015 18(01):91-99.10.1111/ocr.1208325865537 [Google Scholar] [CrossRef] [PubMed]
[14]. Saad MM, Rahman NAA, Mokhtar KI, Bakar NA, Kharuddin AF, Taib WRW, Preliminary study of PAX9 single nucleotide polymorphism (rs8004560) in patients with Class II skeletal base malocclusion contributed by mandibular retrognatismArch Orofac Sci 2018 13(2):112-18. [Google Scholar]