The CML is a clonal haematopoietic stem cell disorder that affects cells of all three lineages. It is a myeloproliferative neoplasm characterised by balanced chromosomal translocation called the Philadelphia (Ph) chromosome. CML progresses in stages termed CP, AP and BC. CML-CP is characterised by leucocytosis with myeloid cell bulge and maintained terminal differentiation accompanied by basophilia and eosinophilia. Blast count is <2% of total leucocyte count. Platelet count may be normal or increased. Marked thrombocytopenia is uncommon in CP [1]. WHO 2016 recommends the defining criteria for CML-AP which are persistent or increasing leucocyte count (>10×109/L), persistent or increasing splenomegaly, persistent thrombocytosis (>1000×109/L), persistent thrombocytopenia (<100×109/L), basophils in peripheral blood ≥20%, 10-19% blast in peripheral blood or bone marrow and additional clonal chromosomal abnormalities in Ph chromosome [1,2]. Presence of >20% blasts in blood or bone marrow or the presence of extramedullary proliferations of blasts defines CML-BC [1,2].
Micromegakaryocytes are observed in peripheral smear in CML, myelodysplasia, polycythemia vera and Hodgkins lymphoma [10,11]. They are dysplastic, small megakaryocytes with increased nuclear to cytoplasmic ratio and hypolobated nuclei. At times seen with budding platelets through the cytoplasm despite being dysplastic. The platelet count is frequently elevated and the platelets on the blood film are often large and dysmorphic. Micromegakaryocytes in peripheral blood of CML patients have been rarely reported in western literature [12-14]. Though very little literature has been found, it is an observational finding that in Indian patients there is increased evidence of circulating micromegakaryocytes in CML [15]. Due to wide lacunae in existing literature, circulating micromegakaryocytes need to be studied in CML patients. The objectives of this study were to assess circulating micromegakaryocytes in different phases of CML and to observe its correlation with clinical and haematological parameters such as splenomegaly, blast count, basophilia and bone marrow fibrosis.
Materials and Methods
This cross-sectional study was conducted in the Department of Pathology and Medicine, Maulana Azad Medical College, Delhi, India after obtaining Ethical Clearance from the Institutional Ethical Committee (IEC) numbered IEC/MAMC/ (80/08/2020/No 257). Ideal sample size using prevalence of micromegakaryocytes in CML as 47.8% was calculated as 95 (Z2pq/L2, Z=1.96, p=prevalence of micromegakaryocytes in CML, q=100-p, L=allowable error 10%). However, due to time and resource constraints 45 newly diagnosed CML patients visiting CML outpatient department were enrolled in the study.
Inclusion criteria: 45 newly diagnosed CML patients visiting CML Outpatient Department were included in the study.
Exclusion criteria: Patients with other myeloproliferative neoplasm and patients of CML on chemotherapy were excluded from the study.
Seventeen patients were included retrospectively and 28 were included prospectively in the study. The diagnostic criteria were as per the WHO 2016, such as leukocytosis with myeloid bulge, with associated cytogenetic and molecular finding of Ph chromosome and or Breakpoint Cluster Region Abelson (BCR ABL) fusion gene by Fluorescence In Situ Hybridisation (FISH) and/or Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) [1]. A detailed history was taken from each patient and thorough clinical examination was done for presence of hepato-splenomegaly. A written informed consent was obtained from all patients for their inclusion in the study. However, consent was waived-off from the patients included in the study retrospectively.
Two ml of peripheral blood was collected in EDTA vial. The EDTA sample was run within 2 hours of collection on an automated haematology analyser (XT 2000i), to obtain complete blood counts including Haemoglobin (Hb), Total Leukocyte Count (TLC), Red Blood Cell Count (RBC), Platelet count (Plt), and calculated Red cell indices and platelets indices. Two peripheral blood smears were made and one was stained with Giemsa. A 200 cell differential count was done on peripheral blood and micromegakaryocytes were identified morphologically and by CD61 (clone-2C9.G3, Thermo Scientific, RTU) immunostaining.
For CD61 immunostaining second Peripheral Blood Smear (PBS) was fixed in cold methanol and ethanol solution in the ratio of 1:1. The slides were then put in a coplin jar filled with 10 mM citrate buffer (pH 6) and heated in microwave for 10 cycles of 3 minutes duration each accompanied by cooling in between 2 cycles which allowed unmasking of epitope. At the end of 10th cycle, 3 washes of PBS buffer was given. Then the slide was incubated overnight with CD61 at 4°C. Incubation with secondary (biotinylated goat anti-polyvalent antibody) and tertiary antibody (peroxidase labelled streptavidin peroxidase complex) was done for 30 minutes each followed by 3 washes of PBS buffer. Freshly prepared Diaminobenzidine (DAB) was applied on the slides and the reaction was monitored under microscope. The slides were counter stained with haematoxylin.
Bone marrow fibrosis was assessed on reticulin stain and was graded according to semi-quantitative bone marrow fibrosis grading system proposed by WHO 2016 [16]. Bone marrow fibrosis was assessed in only 20 cases (due to nonavailability of bone marrow biopsy in all cases).
Statistical Analysis
Data was compiled in Excel and statistical analysis was performed using SPSS software version 24. Continuous variables were presented as mean±SD and categorical variables were presented as percentages. The statistical analysis was done using Pearson chi-square test. The p-value <0.05 was considered statistically significant. Correlation was calculated as Pearson correlation coefficient formula. Pearsons correlation coefficient (R) ranges from -1 to +1. Negative value implies negative correlation while positive value implies positive correlation.
Results
Forty five newly diagnosed patients of CML reporting to Maulana Azad Medical College were included in the study. Of these, 22 were males and 23 females with male to female ratio being 0.95:1. The age of patients ranged from 13 to 70 years with median age being 37 years. At presentation, 27 patients were in CP, 9 in AP and 9 in BC as per WHO criteria laid in blue book 2016 [1,2].
Anaemia (Hb <12 g/dL in females and <13 g/dL in males) was observed in 42 (93%) cases of CML. Out of this, 26 (61.9%) patients were in CP, 7 (16.6%) in AP, 9 (21%) in BC. Hyperleukocytosis (leucocytes count >1 lacs/microliter) was found in 23 (51%) patients. Blast count ranged from 0-7/100 WBCs in CP, 10-15/100 WBCs in AP and 22-45/100 WBCs in BC. There was significant positive correlation of micromegakaryocytes with blast count. (p=0.01, R=0.547). Basophil count ranged from 0-5/100 WBCs in CP, 0-6/100 WBCs in AP and 0-5/WBCs in BC. Thrombocytopenia was seen in 10 (22%) cases while thrombocytosis was seen in 11 (24%) cases of CML. Thrombocytopenia was seen in 3 (11.1%) patients with CP, 1 (11.1%) patient with AP and 6 (66.6%) patients with BC [Table/Fig-1]. Splenomegaly was observed in 43 (95%) patients, out of these 22 patients had moderate splenomegaly and 21 patients had massive splenomegaly. There was no significant correlation of splenomegaly (p=0.41) and basophil count (p=0.79) with micromegakaryocytes [Table/Fig-1].
Clinicohaematological parameters in different phases of CML.
| Phases of CML | Blast Range (/100 WBCs) | Basophil Range (/100 WBCs) | Anaemia (N=42, 93%) | TCP (n=10, 22%) | TCS (n=11, 24%) | LCS>1L (n=23) | Splenomegaly (n=43, 95%) | BM Fibrosis (n=20) |
|---|
| CP (n=27) | 0-7 | 0-5 | 26 | 3 | 10 | 14 | 26 | 9/10 |
| AP (n=9) | 10-15 | 0-6 | 7 | 1 | 1 | 4 | 8 | 4/4 |
| BC (n=9) | 22-45 | 0-5 | 9 | 6 | 0 | 5 | 9 | 6/6 |
TCP: Thrombocytopenia; TCS: Thrombocytosis; LCS: Leucocytosis; BM: Bone marrow
Circulating micromegakaryocytes [Table/Fig-2a,b] were seen in 37 (82%) cases of CML out of which 20 (54%) were in CP, 8 (22%) in AP and 9 (24%) in BC. Twenty out of 27 (74%) patients with CP, 8/9 (89%) patient with AP and 9/9 (100%) patient with BC had circulating micromegakaryocytes. Range of micromegakaryocytes in CP was 0-2/100 WBC, with a mean of 0.57. Circulating micromegakaryocytes increased in AP (Range-0-3/100 WBC, mean-1.7) and BC (Range-0.5-5/100 WBCs, mean-2.6) [Table/Fig-3].
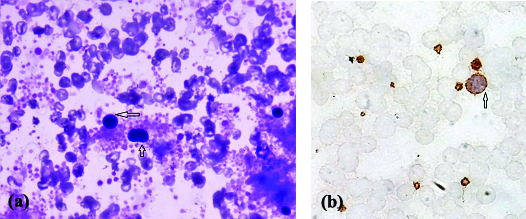
(a) Micromegakaryocytes are seen pinching of dysplastic platelets. (arrow) (Giemsa, 600x). (b) Micromegakaryocytes are seen pinching of dysplastic platelets. (arrow) (CD61, 1000x).
Correlation of circulating Micromegakaryocytes with different phases of CML.
| Phases of CML | Blast range (No./100 WBCs) | Zero mMgk/100 WBCs | 0.5 mMgk/100 WBCs | 1 mMgk/100 WBCs | 2 mMgk/100 WBCs | 3-5 mMgk/100 WBCs | Mean mMgk |
|---|
| CP (n=27) | 0-7 | 7 | 11 | 8 | 1 | 0 | 0.57 |
| AP (n=9) | 10-15 | 1 | 1 | 1 | 5 | 1 | 1.7 |
| BC (n=9) | 22-45 | 0 | 1 | 1 | 3 | 4 | 2.6 |
p=0.001, r-0.547 (Pearson chi-square test)
mMgk: micromegakaryocytes; WBCs: White blood cells
Bone marrow fibrosis was assessed in 20 cases, as biopsy was available in these cases only. Bone marrow fibrosis was detected in 19/20 (95%) cases with CML. Out of these, 9 were in CP with fibrosis ranging between grade 1-2, 4 in AP with fibrosis between grade 1-2, 6 in BC with fibrosis between grade1-3 [Table/Fig-4]. Six (60%) patients with Grade 1 Fibrosis had circulating micromegakaryocytes <0.5, while 7 (78%) patients with Grade 2/3 Fibrosis had ≥1/100 WBCs circulating micromegakaryocytes [Table/Fig-4]. There was significant increase in BM fibrosis as circulating micromegakaryocyte increased (p=0.01 and Pearson correlation=0.543).
Correlation of micromegakaryocytes with bone marrow fibrosis.
| Number of Mmgk/100 WBCs | Number of patients with BMF grade 0 | Number of patients with BMF grade 1 | Number of patients with BMF grade 2 | Number of patients with BMF grade 3 |
|---|
| 0 | 1 | 2 | 0 | 0 |
| <0.5 | 0 | 4 | 0 | 2 |
| 1 | 0 | 2 | 1 | 1 |
| 2 | 0 | 2 | 2 | 1 |
| 3-5 | 0 | 0 | 1 | 1 |
p=0.01, r-0.543 (Pearson Chi-square test)
BMF: Bone marrow fibrosis; Mgk: Megakaryocyte, WBCs: White blood cells
Discussion
Appearance of megakaryocytes in peripheral blood is seen in various diseases like Myelogenous Leukaemia, Polycythemia Vera, myelodysplastic syndrome, hodgkins disease and some cases of sepsis [17]. It was suggested by George Minot for the first time in literature in early 1922 that appearance of megakaryocytes in peripheral blood in Myelogenous Leukaemia indicates an impending change in character of the disease [18].
Circulating micromegakaryocytes were seen in 82% cases of CML. Anand M et al., found circulating micromegakaryocytes in 47.8% patients. In this study, 74% patients with CP, 89% patients with AP and 100% patient with BC had circulating micromegakaryocytes. In a study by Anand M et al., 106/235 (45.1%), 41/65 (63.1%) and 8/24 (33.3%) patients in CP, AP and BC phases had circulating megakaryocytes. Present study shows that there is a significant increase in circulating micromegakaryocytes with increase in blast count (p<0.01 and Pearson correlation=0.547). Median value of micromegakaryocytes in CP, AP and BC was 0.5, 2 and 3. Median values of micromegakaryocytes in the study by Anand M et al., was 0.75% for CP, 2.0% for AP and 6.0% for BC, which is quite similar to present study [15].
Megakaryocytes in the peripheral blood appears slightly different from that present in bone marrow. Nucleus of the megakaryocytes present in peripheral blood gets stripped off from their cytoplasm, however some cytoplasm remains which are in process of differentiation towards platelets. Thus, in circulation micromegakaryocytes are seen pinching-off dysplastic platelets from the surface [Table/Fig-2a,b] [11]. In bone marrow, the megakaryocytes have abundant cytoplasm with pseudopods whose segmentation results in the formation of platelets. Nucleus in the bone marrow is more vesicular and lobulated. The nucleus of megakaryocytes seen in the peripheral blood are smaller and are of various shapes and sizes varying from mono nuclear to binuclear and stains very deeply and are hyperchromatic [17].
Anand M et al., described the morphology of circulating micromegakaryocytes in CP and BC of CML. In CP, the micromegakaryocytes are mature and small, lymphocyte-like cells with non-lobulated nucleus and scant cytoplasm. Platelets budding from the cytoplasm can be seen. However in BC, these micromegakaryotes are large lobulated granular or agranular cytoplasmic masses. These are connected by narrow bridges of cytoplasm coming-off from blasts. As the micromegakaryocytes mature, there is decrease in nuclear size and deepening of chromatin condensation [15].
It is advisable that all patients of CML should be screened routinely for circulating micromegakaryocytes in peripheral smear so as to predict their behaviour especially in Indian backdrop as patients in Indian settings show more pronounced leukocytosis, marrow fibrosis and behave in different manner than their western counterpart [15]. This is especially more important in resource constrained centers where molecular and flowcytometric follow-up of these patients is a limiting factor as this needs just morphological training. Megakaryocytes in the peripheral blood have been reported in very few studies [15,19]. However, megakaryocytic BC has been reported in some studies and case reports [20].
Limitation(s)
This study is limited due to its small sample size. CD61 immunostaining could not be done in all cases as few cases were collected retrospectively. Bone Marrow fibrosis was only studied in 20 cases and longitudinal data could not be assessed so as to more conclusively follow these patients for longer duration.
Conclusion(s)
Micromegakaryocytes and cytoplasmic fragments of micromegakaryocytes are found in peripheral blood in CML. With progressive phase and increase in blast count and marrow fibrosis circulating micromegakaryocytes increase in circulation thus predating the outcome of disease in substantial manner. This is in contrast to western data where micromegakaryocytes are not so profound in blood. However, a study on large number of subjects with longitudinal follow-up is needed for better correlation.
TCP: Thrombocytopenia; TCS: Thrombocytosis; LCS: Leucocytosis; BM: Bone marrow
p=0.001, r-0.547 (Pearson chi-square test)
mMgk: micromegakaryocytes; WBCs: White blood cells
p=0.01, r-0.543 (Pearson Chi-square test)
BMF: Bone marrow fibrosis; Mgk: Megakaryocyte, WBCs: White blood cells