Port Site Metastasis following Laparoscopic Excision of Ovarian Carcinosarcoma
Anju Singh1, Avir Sarkar2, Rimpi Singla3, Nalini Gupta4, Bhavana Rai5
1 Assistant Professor, Department of Obstetrics and Gynaecology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
2 Junior Resident, Department of Obstetrics and Gynaecology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
3 Assistant Professor, Department of Obstetrics and Gynaecology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
4 Professor, Department of Cytology and Gynaecological Pathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
5 Professor, Department of Radiotherapy, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Avir Sarkar, House Number 459, Sector 15A, Chandigarh-160015, Chandigarh, India.
E-mail: avirsarkar93@gmail.com
Port site metastasis is a rare complication and carcinosarcoma is itself a rare malignant tumour of the ovary. Hereby, Authors report a case of an ovarian carcinosarcoma which was retrospectively diagnosed from the metastasis which developed at the specimen retrieval port site of primary laparoscopic surgery. A 48-year-old nulliparous lady underwent laparoscopic cystectomy previously for endometrioma. Six months postlaparoscopy, she developed pain and palpable mass at the trochar site. Fine Needle Aspiration Cytology (FNAC) showed metastatic adenocarcinoma of ovarian origin confirmed by immunohistochemistry. Six cycles of chemotherapy followed by total hysterectomy, resection of the mass and attached tubal segment, opposite salpingo-oophorectomy and omentectomy was done. Post-surgery, patient was followed-up with three chemotherapy sessions. Final histopathological report showed carcinosarcoma of ovary at port site with omental deposit.
Gynaecology, Minimally invasive surgery, Neoplasia
Case Report
A 48-year-old nulliparous lady presented to Gynaecology Out Patient Department of our institute with complaint of dull lower abdominal pain and a palpable nodular lump in the laparoscopic trocar site of right anterior iliac spine for two months. She had undergone laparoscopic right ovarian cystectomy for persistent lower abdomen pain and right adnexal cystic mass six months back. Histopathological report of surgical specimen was suggestive of endometriosis. Currently, she was not taking any medication or hormonal pills.
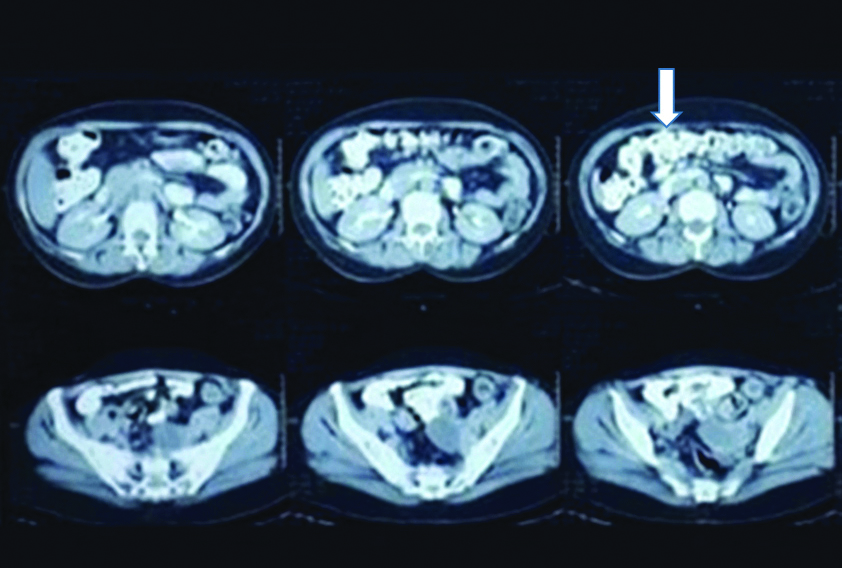
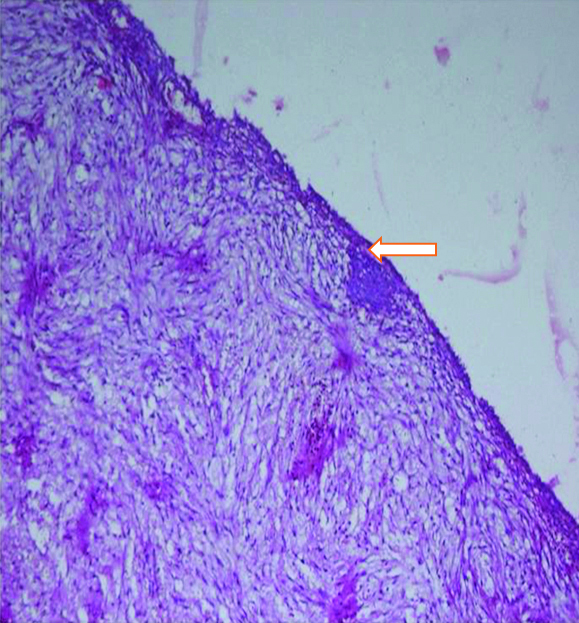
There was no history of loss of appetite, loss of weight, bladder, bowel or menstrual irregularity. On examination, a 3×4 cm firm nodule was present at laparoscopy port site scar. Per speculum and per vaginal examinations was unremarkable. Contrast Enhanced Computed Tomography (CECT) of abdomen showed a well-defined 3.2×3.3×3.2 cm solid mass in the anterior abdominal wall involving the right rectus abdominis, external and internal oblique and transverse abdominis muscles. Another focal 2.7×2.1×1.6 cm solid mass was seen in the vesico-uterine space abutting the adjacent urinary bladder wall and anterior uterine myometrium. Bilateral ovaries were normal [Table/Fig-1]. Upper and lower gastrointestinal endoscopy were unremarkable. Histopathological slide review of previous cystectomy specimen at our institute revealed normal ovarian parenchyma [Table/Fig-2].
CECT films showing solid mass in the anterior abdominal wall involving muscles of the anterior abdominal wall with omental thickening. Another mass (marked with white arrow) is seen involving the vesico-uterine space.
Section shows a cyst wall (marked by a white arrow) comprised of fibro-collagenous tissue focally lined by flattened lining epithelium (H&E x20X).
Fine Needle Aspiration Cytology (FNAC) of the port site nodule suggested metastatic adenocarcinoma. Immunohistochemistry showed strong cytoplasmic positivity with vimentin, patchy nuclear positivity with PAX 8, strong membranous positivity with Cytokeratin-7 (CK-7) suggested either ovarian or mullerian origin. Differentials at this point were either serous or endometrial epithelial ovarian neoplasms with interposed mesenchymal components.
In view of advance stage, she was prescribed with 6 cycles of paclitaxel and carboplatin followed by surgical excision. Intraoperatively a 2×1×1 cm whitish nodular tissue stuck to the posterior surface of the uterus was identified. Total abdominal hysterectomy, left salpingo-oophorectomy, infracolic omentectomy along with 2 cm circumferential free margin local excision of the port site nodule was done.
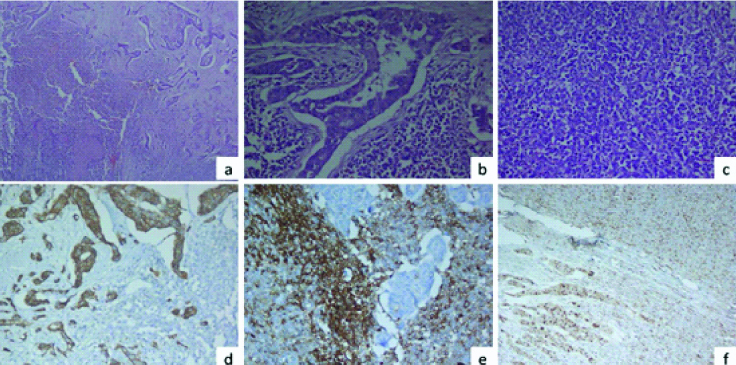
Histopathology of excised port site mass showed a biphasic tumour with glandular component mainly arranged in complex glands lined by atypical cells and background showing stromal component with small round cells with prominent capillaries, suggestive of carcinosarcoma. Omentum and tissue attached to posterior surface of uterus also had foci of similar atypical glands on its surface while uterus, left fallopian tube and ovary were free of tumour. Immunohistochemistry of surgical specimen showed both epithelial components diffusely positive for CK-7 and sarcomatous component membrano-cytoplasmic dot like positivity for CD10 [Table/Fig-3]. Both components showed focal nuclear positivity for cyclin D1 and negative for Oestrogen Receptor (ER), Progesterone Receptor (PR), Desmin, Smooth Muscle Antibody (SMA) and Paired Box Protein (PAX8). Post-surgery, she was prescribed with 3 cycles of chemotherapy. At present she is on regular follow-up and doing well.
A panel of microphotographs of a case of carcinosarcoma at port site; a) A biphasic tumour comprising of epithelial component and mesenchymal component with areas of necrosis (H&E x20X); b) Epithelial component in the form of complex glandular pattern (H&E x40X); c) Mesenchymal component comprising of sheets of small round cells resembling endometrial stromal sarcoma (H&E x 40X); d) CK 7 positivity in the epithelial component (IHC CK7 x40X); e) CD10 positivity in the mesenchymal component (IHC CD10 x40X); f) Cyclin D1 nuclear positivity in the epithelial as well as mesenchymal component (H&E x40X).
Discussion
The reported incidence of Port Site Metastasis (PSM) in gynaecological oncosurgeries is 0.14 to 1.8% [1-3]. Ovarian carcinosarcoma is responsible for 1-3% of all ovarian malignancies worldwide [4]. No separate incidence from India has been reported so far. There may be various sites of origin like ovary, fallopian tube, uterus, urethra and breast. It may involve either the mesenchymal or the epithelial component of the affected organ [5]. The epithelial component may be endometrioid, squamous, clear cell or serous type and the mesenchymal component can be homologous in the form of leiomyosarcoma or fibrosarcoma or heterologous in the form of osteosarcoma or rhabdomyosarcoma [6]. The average age of onset is during the sixth and seventh decades and most patients present in advanced stages [1,2]. Patient in the present study was in fifth decade with advanced stage. Almost 50% of patients have pelvic and para-aortic lymph nodes at presentation. Median age of survival after diagnosis is less than 2 years [2]. Negative prognostic factors include sarcomatoid component exceeding 25% of total tumour bulk, advanced stage or higher grade at presentation, large size tumour, more than 65 years of age, presence of p53 mutation and history of long-term tamoxifen use [6].
Port Site Metastasis (PSM) following laparoscopic surgical excision of ovarian carcinosarcoma is very rare. Free cancer cells are able to implant on raw surfaces including damaged peritoneal surfaces. A number of proposed hypothesis to explain the development of PSM includes direct seedling of tumour cells from specimen during retrieval or spread via aerosolisation caused by “chimney” effect during insufflations of pneumoperitoneum [7]. The cause of PSM in present patient can be attributed to direct seedling effect due to lack of use of endobag during primary surgery. Rinsing of the port site with betadine following primary surgery could also have prevented PSM in index case.
Evidence also suggests that microtrauma caused at port insertion sites alter the immunological defences of the epithelium thereby inducing metastatic changes [7]. Pneumoperitoneum using Carbon Dioxide (CO2) might trigger atypia in subcutaneous tissue. CO2 being an acidic gas decreases the intra-abdominal pH and forces the cells to translate into anaerobic metabolism thereby activating enzymes that lead to increase in cellular mitosis and tissue proliferation [7].
There are multiple ways to prevent PSM that includes reducing desufflation and inadvertent gas leaks, using frequent intra-abdominal lavage, rinsing trocar sites with 5% povidone iodine solution before closure, deflating the abdomen with trocars in place and closure of 10 mm or bigger ports in layers [8], Almost 71% of PSM occurs at tissue manipulating port only. So, protected tissue retrieval alone can reduce a vast majority of port site malignancies. It is preferable to remove ovarian cyst tissue in endo bag/any bag especially if done for endometriosis to avoid port site pathology. Although studies on rat models have demonstrated protective role of helium insufflation in decreasing PSM but CO2 is still the preferred gas because of the increased risk of lethal gas embolisms associated with helium.
Several authors have shown that PSM is usually indicative of advanced disease. It is generally present in the setting of disseminated disease and indicate a poor prognosis. The interval between laparoscopy in gynae-oncosurgeries and detection of PSM is a significant predictor of survival [9]. PSM detected before 7 months after primary laparoscopic surgery carries a median survival of 12 months compared to 37 months in whom PSM was detected after 7 months of surgery. Index case has recurrence within 6 months. Consolidated data from 18 registries from 1998 to 2009 showed that ovarian carcinosarcomas with a mean survival of only 8-26 months have worse 5-year disease specific survival rate compared to high grade papillary serous carcinoma of ovary.
GOG 261 phase III trial showed that complete cyto-reductive surgery with no gross residual disease is associated with improved outcome in ovarian carcinosarcoma. There is very limited role of cytotoxic chemotherapy and so maximal surgical effort should be done to achieve an R0 resection. There are varying opinions regarding the choice of adjuvant chemotherapy, however there is an increasing trend towards the use of platinum-based therapy including paclitaxel and carboplatin [10]. National Comprehensive Cancer Network (NCCN) guidelines recommend surgical excision of resectable metastasis followed by consideration of adjuvant chemotherapy or radiotherapy.
Since laparoscopic tumour resection is now-a-days the first choice in the treatment of benign ovarian tumours, it is mandatory to pre-operatively differentiate benign from malignant ovarian neoplasms. The problem occurs in the case of early ovarian cancers which are very difficult to detect pre-operatively and can lead to unwelcome consequence in the form of port site metastasis as in this patient.
Conclusion(s)
As port site metastasis is complication of laparoscopic surgery with poor prognosis, steps should be taken like specimen retrieval in endobag, reducing desufflation and inadvertent gas leaks, using frequent intra-abdominal lavage, rinsing trocar sites with 5% povidone iodine solution before closure.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: July 07, 2020
Manual Googling: Nov 11, 2020
iThenticate Software: Dec 11, 2020 (7%)
[1]. Lönnerfors C, Bossmar T, Persson J, Port-site metastases following robot-assisted laparoscopic surgery for gynecological malignanciesActa Obstet Gynecol Scand 2013 92(12):1361-68.10.1111/aogs.1224523968434 [Google Scholar] [CrossRef] [PubMed]
[2]. Palomba S, Falbo A, Russo T, La Sala GB, Port-site metastasis after laparoscopic surgical staging of endometrial cancer: A systematic review of the published and unpublished dataJ Minim Invasive Gynecol 2012 19(4):531-37.10.1016/j.jmig.2012.03.02322748961 [Google Scholar] [CrossRef] [PubMed]
[3]. Zivanovic O, Sonoda Y, Diaz JP, Levine DA, Brown CL, Chi DS, The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant diseaseGynecol Oncol 2008 111(3):431-37.10.1016/j.ygyno.2008.08.02418929404 [Google Scholar] [CrossRef] [PubMed]
[4]. Pankaj S, Nazneen S, Kumari A, Kumari S, Choudhary V, Roy VK, A rare tumour of the ovary: Carcinosarcoma report and review of literatureJ Obstet Gynaecol India 2016 66(Suppl 2):648-50.10.1007/s13224-015-0788-427803534 [Google Scholar] [CrossRef] [PubMed]
[5]. Makris GM, Siristatidis C, Battista MJ, Chrelias C, Ovarian carcinosarcoma: A case report, diagnosis, treatment and literature reviewHippokratia 2015 19(3):256-59.10.1155/2015/42974025737786 [Google Scholar] [CrossRef] [PubMed]
[6]. Yalcin I, Meydanli MM, Turan AT, Taskin S, Sari ME, Gungor T, Carcinosarcoma of the ovary compared to ovarian high-grade serous carcinoma: Impact of optimal cytoreduction and standard adjuvant treatmentInt J Clin Oncol 2018 23(2):329-37.10.1007/s10147-017-1215-x29143144 [Google Scholar] [CrossRef] [PubMed]
[7]. Kadi N, Isherwood M, Al-Akraa M, Williams S, Port-site metastasis after laparoscopic surgery for urological malignancy: Forgotten or missedAdv Urol 2012 13(2):60953110.1155/2012/60953122611383 [Google Scholar] [CrossRef] [PubMed]
[8]. Zhang Y, Luo X, Fan B, Chen H, Fu A, Huang J, Effect of CO2 pneumoperitoneum on the proliferation of human ovarian cancer cell line SKOV-3 and the expression of NM23-H1 and MMP-2Arch Gynecol Obstet 2015 291(2):403-11.10.1007/s00404-014-3414-225141992 [Google Scholar] [CrossRef] [PubMed]
[9]. Kruitwagen RF, Swinkels BM, Keyser KG, Doesburg WH, Schijf CP, Incidence and effect on survival of abdominal wall metastases at trocar or puncture sites following laparoscopy or paracentesis in women with ovarian cancerGynecol Oncol 1996 60(2):233-37.10.1006/gyno.1996.00318631544 [Google Scholar] [CrossRef] [PubMed]
[10]. Rauh-Hain JA, Starbuck KD, Meyer LA, Clemmer J, Schorge JO, Lu KH, Patterns of care, predictors and outcomes of chemotherapy for uterine carcinosarcoma: A National Cancer Database analysisGynecol Oncol 2015 139(1):84-89.10.1016/j.ygyno.2015.08.01426307402 [Google Scholar] [CrossRef] [PubMed]