Introduction
Contamination of heavy metals in the environment is a major global concern, because of toxicity and threat to the human life and ecosystem. The levels of metals and its toxicity is increasing in all states of environments including air, water and soil. Metal contaminated environments pose serious threat to health and ecosystems. One of the earliest metals discovered by the human population is Lead (Pb). It has a number of unique properties such as low melting point, ductility, high malleability, resistance to corrosion and low cost. This has made its widespread usage in different industrial sectors, which in turn has led to its manifold occurrence in free form in the environment. During that time hazardous effects of lead was not considered, but recent years the side effects of lead toxicity become worst. At the same time, because of its non biodegradable nature, it is considered as potent hazardous toxins and causes serious health issues to people. There are a number of reports evidencing this aspect. One such report is the incidence of childhood lead poisoning, it is mainly due to drinking contaminated water in West Bengal and Bangladesh [1]. The use of lead and its toxicity in the urban and rural areas has become a national calamity when compared to its occurrence in Himalayan population with no industrial lead exposure.
History Behind Usage of Lead
The use of lead by humans dates back to thousands of years to the times of Romans, Egyptians and Babylonians for making statues, coins, water pipes and weights. It was not used for ornamental usage because of its soft nature. They used lead compounds to glaze containers used for food and water, boil and condense grape juice in lead pots for pre serving and sweetening of wine [2]. One of the major sources of lead exposure are from lead acid batteries, cosmetics, leaded gasoline and paints. The usage of leaded gasoline was banned in US from 1970, followed by 1975 many countries including Western Europe, Korea, Thailand, Australia, China, Vietnam, the Philippines, Japan, Canada, Mexico, Central and South America stopped using this of leaded gasoline [3]. India banned the use of leaded petrol by 2000.
Sources of Lead Exposure
Domestic Environment
Lead is ubiquitous in the environment, the contribution sources includes both natural and human activities. Human activity includes smelting, refining and mining, this may results in lead concentration in the environment.
Food: Lead present in the environment may get deposited in the growing plant or food processing and results in lead contamination, few reasons are given below:
Lead present in the pesticide, fertilizer or soil may taken up into a root and gets deposited in leaf.
Lead from industrial origin may get deposited in the plant.
Canned foods are the source of lead which is leached from the solder in the seams of can.
Leaching of lead from vessels like lead glazed ceramic or porcelain pots.
Drinking water: Usage of water pipes which are made up of lead may contaminate the drinking water and increases the blood lead level who consumes it.
Air: Lead in the air comes from various sources. Largest contribution through leaded gasoline. Highest concentration observed near smelters.
Lead based paint: Lead has been used as a pigment and drying agent in primers, paints and resigns. Its usage was banned in 1970’s. The usage of lead paints was banned for residential purpose, but still older building that were already painted with leaded paint may cause lead exposure in young children [4].
Occupational Environment
Occupational and environmental exposure [Table/Fig-1] to lead may resulting in serious health issues in developing countries [5].
Industries and occupation associated with lead exposure.
| Major occupations and industries associated with lead over exposure |
|---|
Battery manufacturing Construction workers Demolition workers Foundry workers Gas-station attendants Gasoline additives Lead miners Lead smelters and refiners
| Pigment manufacturing Pipe fitters Plastics industry Pottery workers Radiator repair Rubber industry Soldering of lead products Welders
|
Absorption, distribution and Excretion of Lead
Absorption
Routes of absorption of Lead (Pb) is through ingestion, inhalation or through skin. Exposure to lead occurs mainly through gastrointestinal (GI) tracts and respiratory system [6]. Absorption is through respiratory system mainly dependents on size of the particle. It is estimated that be 30-40% of inhaled lead reaches the bloodstream [7]. Absorption rate through GI tract also depends on the age and nutritional status of the exposed individual. The average percentage of lead absorption in adults is 10 to 15% of the ingested quantity whereas it increases upto 50% in infants, young children and pregnant women. The percentage of lead absorption increases in fasting state and in the deficiency of calcium, iron, phosphorus or zinc [8]. Iron leads to defective absorption of lead, hence, Iron deficiency leads to increased concentration of blood lead in children [9]. Researchers demonstrated that increased intake of calcium supplementation in infants and children results in decreased absorption of lead [10]. High intake of magnesium, phosphate and dietary fat leads to decrease gastrointestinal absorption of lead [11]. Lead absorption occurs through both passive and facilitated diffusion [12]. Some studies evidences the hypothesis that divalent metal transporter 1 (DMT1) is responsible for lead transport [13].
Inorganic form of Pb from food, water, paint, vinyl products and tetraethyl lead from leaded gasoline are absorbed through the skin [14]. Incase of lead absorption through skin, it is first transported into the plasma and rapidly concentrated into the extra cellular fluid pool like sweat and saliva without significant uptake by erythrocytes.
Distribution of Lead
After absorption lead gets accumulate in blood, soft tissues and bone. Approximately 99% of the absorbed lead is found in the erythrocytes, 1% is left in plasma and serum. The kinetics of lead transfer from blood to soft tissues which tends to be low and takes approximately 4 to 6 weeks. Because of the short half-life of 35 days in the blood, this blood lead level cannot be used to diagnose the lead exposure happened 6 weeks before [15]. Higher percentage of lead is taken up by the kidney followed by liver and other soft tissues [16]. Half life of lead in various organis varies, in blood the half-life is 35 days in blood [17]. In soft tissues half-life was found to be 40 days and in bones it was 20 to 30 years [18].
Lead distribution in various organs depends on the blood flow to the tissues. It can crosses the blood brain barrier [19]. Various studies revealed that oral intake of inorganic lead affect the immune system.
Excretion of Lead
Inorganic lead does not metabolised in our body, which is excreted unchanged in urine. The mechanisms by which the absorbed lead excreted appear unclear. Lead Excreted by many ways which includes secretion into the bile, gastric fluid, and saliva [20]. Alkyl lead like tetraethyl and tetramethyl lead on oxidative dealkylation form a highly neurotoxic compounds [21]. This reaction is catalysed by cytochrome p450-dependent monooxygenase enzyme present in liver [22]. Other routes of excretion of lead includes nails and sweat [23,24]. On the whole, lead is excreted very slow and tends to accumulate in the body with biological half-life of 10 years. Lead is also excreted in milk in concentrations of upto 12 μg/L.
Children are more vulnerable to lead toxicity due to the following reasons:
Frequent hand to mouth activity.
Children absorb 40 to 50% of dietary lead whereas adult absorb only 10%.
Nervous systems are rapidly developing in children.
Lead in kidney interfere with vitamin D 1,2 dihydroxy cholecalciferol.
Lead interferes with the formation of active vitamin D, thereby interfere with calcium absorption.
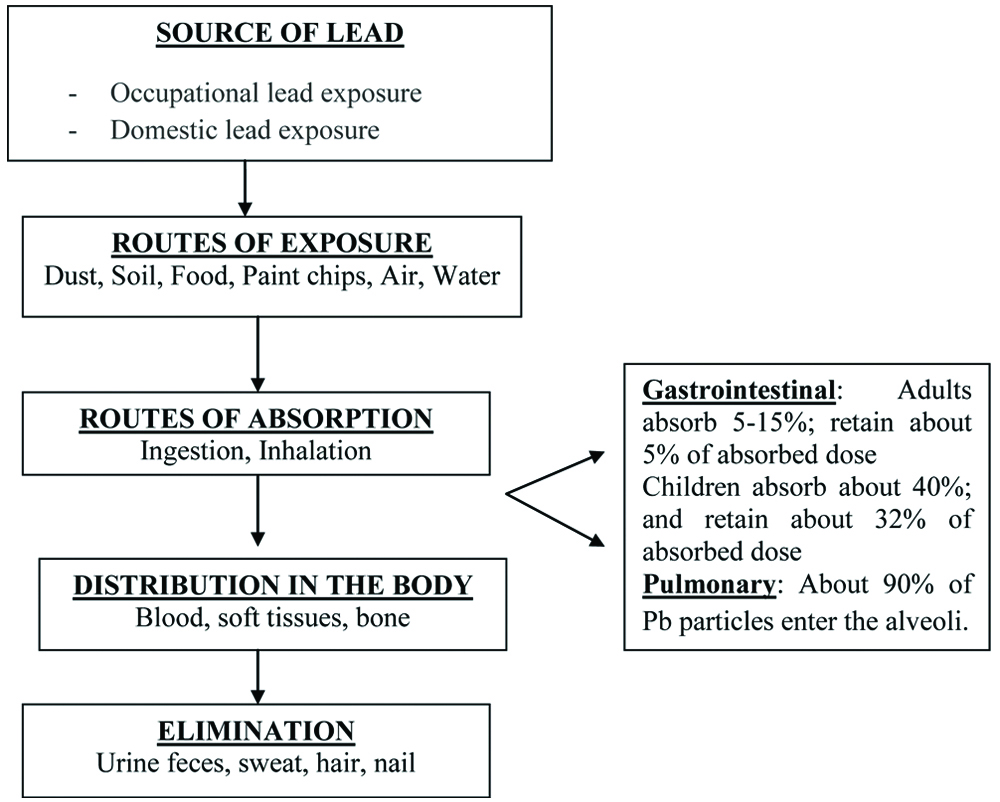
The absorption, distribution and excretion of lead is presented in [Table/Fig-2].
Diagrammatic representation showing the source of exposure, absorptions, distribution and excretion of lead.
Biochemical Indicators of Lead toxicity
Generally Blood Lead Level (BLL), Blood δ Amino Levulinic Acid Dehydratase (ALAD) activity, Urinary Amino Levulinic Acid (ALA), Eythrocyte Protoporphyrin level and creatinine levels are measured to evaluate lead toxicity.
Toxic Signs and Symptoms of Lead Toxicity
Blood Lead Level (BLL) of 60 μg/dL was considered safe during 1960s. In 1985, the acceptable level was reduced to 25 μg/dL and it was further reduced to 10 μg/dL in 1991 [25,26,27]. The World Health Organisation (WHO) lead guidelines recommended tolerable lead intake level based from review of the scientific evidence conducted in 2010, the Joint Food and Agriculture Organisation of the United Nations estimated the safer weekly intake 25 μg/kg body weight.
The following [Table/Fig-3] describes the toxic effect of lead in various organs in adults and children.
Toxic effects of lead in different organs in children and adults.
| Toxic effects of lead in adults |
|---|
| Toxic effects | Blood Pb level (in pb/dL) |
|---|
| Nervous system: Overt clinical encephalopathy | 100-120 |
| Kidney: Atrophy and interstitial nephritis | 40-100 |
| Gastrointestinal: Colic | 40-60 |
| Blood cells: Anemia | 50 |
| Nervous system: Learning/IQ disruption, sensory system deficits | 40 |
| Heart and blood vessels: Hypertension | <7 |
| Biochemical: Enzymes changes | 3-30 |
| Toxic effects of lead in children |
| Toxic effects | Blood Pb level (in pb/dL) |
| Kidney: Atrophy and interstitial nephritis | 80-120 |
| Nervous system: Clinical encephalopathy | 80-100 |
| Gastrointestinal: Colic | 60-100 |
| Blood cells: Anaemia | 20-40 |
| Biochemical changes: Enzymes level altered | <10 |
| Nervous system: IQ disruption, sensory system deficits | <10 |
Mechanism of Lead-Induced Toxicity
Though various mechanisms postulated about lead induced toxicity, the mechanism represented in [Table/Fig-4] was considered to be most important mechanism which involves oxidative stress. Enormous number of evidences have shown that lead induced generation of reactive oxygen species resulted in oxidative stress and weakens the cells defense mechanism [28].
Schematic representation of various mechanisms of lead toxicity.
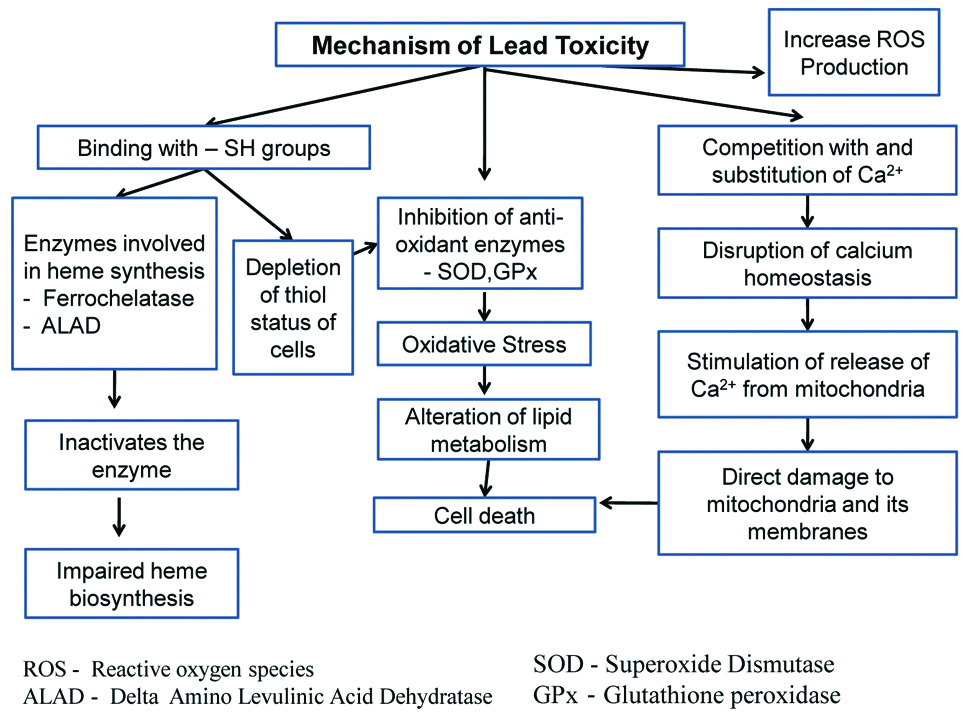
There is an important indirect mechanism also which involves the depletion of cells’ major sulfhydryl resulting in oxidative stress [29]. When Glutathione (GSH) is depleted in the body by lead, the body starts making more GSH from cysteine. This antioxidant defense mechanism may be protected by many enzymes. The cofactors like selenium, zinc, copper of many enzymes may be replaced by lead, and thereby, resulting in enzyme inactivation. Various studies in lead-exposed animals reported to have either elevated lipid peroxidation or decreased intrinsic antioxidant defense in various tissues.
Oxidative Stress
Superoxide Dismutase (SOD), a free radical scavenger and metalloenzyme (zinc/copper) [30]. Various research revealed that lead exposure significantly decreased the level of SOD. This may be due to an increase in lead concentration in these tissues and their possible reaction with this enzyme thereby, reducing the disposal of superoxide radicals. Catalase is an efficient decomposer of H2O2 and known to be susceptible to lead toxicity. Lead induced decrease in brain Glutathione Peroxidase (GPx) activity may arise as a consequence of impaired functional groups such as GSH and Nicotinamide Adenine Dinucleotide Phosphate (NADPH) or selenium mediated detoxification of toxic metals. While antioxidant enzyme Glutathione S-transferase (GST) is known to provide protection against oxidative stress [31].
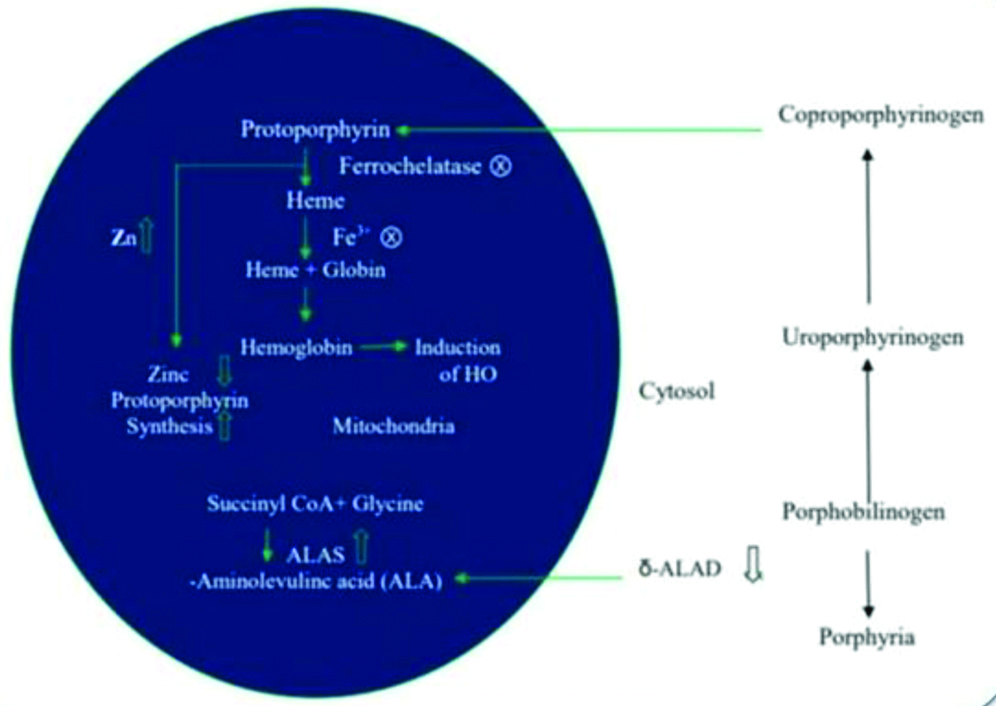
Lead reaction with oxyhaemoglobin results in superoxide radical formation. 5-ALAD is involved in the formation of heme precursor (porphobilinogen) by the condensation of two S-aminolevulinic acid (ALA). Hence, the ALAD inhibition results in the impairment of heme synthesis, and resulting in accumulation of ALA [Table/Fig-5]. Accumulation of ALA undergo metal catalysed autooxidisation, resulting in the conversion of oxyhaemoglobin to methemoglobin. This conversion results in the formation of ROS like superoxide and hydroperoxides.
Lead effects in haemoglobin synthesis pathway.
ALAD: Amino levulinic acid dehydratase
Current Treatment Strategy for Lead Toxicity
Some common chelating agents used against lead poisoning are given below:
Calcium disodium ethylene diamine tetraacetic acid (CaNa2EDTA)
D-penicillamine
Meso 2,3-dimercaptosuccinic acid (DMSA)
Sodium 2,3-dimercaptopropane-1-sulphonate (DMPS)
Limitations of Current Chelating Agents
Treatment with DMSA and DMPS has got lesser adverse effects given in [Table/Fig-6]. Most of the conventional have side effects like reducing essential element level in the body. The Centers for Disease Control and Prevention (CDC) recommend chelating agent only when the blood lead level goes beyond 45 μg/dL. So, there is always a need for an alternative treatment with no side effects.
Limitations of current chelating agents.
| Chelating agents | Limiting factors |
|---|
| CaNa2EDTA | Cannot pass through cellular membrane, use is restricted to ECF |
| Produce renal toxicity |
| Diuresis of endogenous zinc, hence monitoring is essential |
| D-penicillamine | Cause anaphylactic reaction in patient allergic to penicillin |
| In children-monitoring with blood count, urinanalysis |
CaNa2EDTA: Calcium disodium ethylene diamine tetraacetic acid; ECF: Extracellular fluid
Newer Research Approach Against Lead Toxicity
Role of Antioxidant in Lead Toxicity Treatment [
33]
In recent days, attention on usage of herbal drugs is increasing. Many plant products are rich source of antioxidant and can be used to prevent oxidative stress. As number of synthetic antioxidants has shown to have side effects, thus there has been increasing interest in using plant extract. Crude extracts of many plants observed to modify the toxic effect of lead, it is mainly due to antioxidant properties of flavonoids present in plants. Currently, antioxidants are reported to have vital role in the treatment of lead poisoning in humans as lead induces toxicity in various organs like brain, kidney and Liver.
Lead induced Liver Toxicity
Environmental exposure to pollutant like lead will affect and produce damage to the liver by various mechanism, mainly due to oxidative stress. Considering the mechanisms have been explicitly defined, oxidative stress was found to be one of the important mechanisms involved in toxic effects of lead. Hepatotoxicity is the injury to the liver that results in the impaired function of liver caused by the exposure to a drug or environmental xenobiotics. It may be due to lipid peroxidation, reduced glutathione and overproduction of ROS [34].
Role of Herbal Extracts in Lead induced Hepatic Damages [
35-
49]
Numerous studies have been done to find the effect herbal product against lead induced damages. The literature below [Table/Fig-7] shows the protective effect of various plant extract against lead induced hepatic damages. The hepatoprotective effects produced by these plants are may be due to the presence of secondary metabolites, polyphenolic compound flavonoids.
Herbal research on lead induced heapatic damage [35-49].
| Plant name | Inducing agent and dosing | References |
| Cayratia carnosa | Lead acetate - 20 mg/Kg/b.wt/i.p for one day | Suganthi V et al., 2013 [35] |
| Tinospora cordifolia | Lead nitrate - 5 mg/kg/B.W/oral for 30 days | Sharma V and Pandey D, 2010 [36] |
| Asparagus racemosus | Lead nitrate - 20 mg/Kg body weight/oral for 45 days | Sharma VE et al., 2012 [37] |
| Leucas aspera | Lead acetate - 50 mg/kg/ b.wt/oral for 21 days | Thenmozhi M et al., 2013 [38] |
| Zingiber officinale Roscoe | Lead acetate -500 ppm by oral 50 for days | Attia AM et al., 2013 [39] |
| Spirulina | Lead acetate - 1.89 mg/kg for 7 days | Hemalatha K et al., 2012 [40] |
| Coriandrum sativum | Lead nitrate - 40 mg/kg/b.wt/oral for 7 days | Kansal L et al., 2011 [41] |
| Ocimum sanctum linn | Lead acetate - 2.10 mg/150 g/b.wt/oral for 3 days | Akilavalli N et al., 2011 [42] |
| Vitis vinifera | Lead acetate - 100 mg/kg/b.wt/ip - 7 days | Abeer M, 2012 [43] |
| Turmeric and myrrh | Lead acetate - 0.5% oral- 8 weeks | El-Ashmawy IM et al., 2006 [44] |
| Curcuma longa | Lead acetate - 1000 mg/kg/b.wt/oral for 28 days | Baxla SL et al., 2013 [45] |
| Morocco carob honey | Lead acetate - 2 g/kg.b.wt/oral for 24 days | Fihri AF et al., 2016 [46] |
| Green tea | Lead acetate - 0.4% oral for 8 weeks | Mehana EE et al., 2012 [47] |
| Murraya koenigii | Lead acetate - 15 mg/kg/b.et/i.p for 7 days | Ghosh DE et al., 2013 [48] |
| Moringa oleifera | Lead acetate - 2000 ppm for 2 weeks after that 7 days drug treatment | Velaga MK et al., 2014 [49] |
Conclusion(s)
Lead is an environmental pollutant. It reaches environment through the deterioation from lead based paints, batteries and business that involves lead. When blood lead level reaches more than 10 microgram/dL, it produces toxicity. The signs and symptoms varies depends upon the dose of exposure. It affects the Nervous system, Renal system, Endocrine glands, Blood, Gastrointestinal tract, Cardiovascular system and Reproductive system. The major mechanism for lead toxicity is due to increased production of reactive oxygen species and inhibition of enzyme action (Lead binds to enzymes with sulphydryl group). Chelating agents like DMSA-2,3 dimercapto succinic acid and monoisoamyl DMSA can be used against lead toxicity. But, the main disadvantage of chelators is that, they are toxic in nature and it cannot completely remove lead from all tissues. Newer trend in treating lead toxicity is by using natural antioxidants. Antioxidant potential of herbal products can have beneficial effect on treating lead induced toxicity. This review will help young researchers to start more works on lead related toxicity study.
[1]. Kalia K, Flora SJ, Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoningJournal of Occupational Health 2005 47(1):01-21.10.1539/joh.47.115703449 [Google Scholar] [CrossRef] [PubMed]
[2]. Patrick L, Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatmentAltern Med Rev 2006 11:02-22. [Google Scholar]
[3]. Tzanakakis GN, Tsatsakis AM, Lead toxicity update. A brief reviewMed Sci Monit 2005 11(10):RA329-36. [Google Scholar]
[4]. Dixon SL, Wilson JW, Clark CS, Galke WA, Succop PA, Chen M, Effectiveness of lead-hazard control interventions on dust lead loadings: Findings from the evaluation of the HUD lead-based paint hazard control grant programEnvironmental Research 2005 98(3):30310.1016/j.envres.2005.02.00215910785 [Google Scholar] [CrossRef] [PubMed]
[5]. Yucebilgic G, Bilgin R, Tamer L, Tükel S, Effects of lead on Na+-K+ ATPase and Ca+ 2 ATPase activities and lipid peroxidation in blood of workersInternational Journal of Toxicology 2003 22(2):95-97.10.1080/1091581030509612745990 [Google Scholar] [CrossRef] [PubMed]
[6]. Wani AL, Ara A, Usmani JA, Lead Toxicity- A reviewInterdiscip Toxicol 2015 8(2):55-64.10.1515/intox-2015-000927486361 [Google Scholar] [CrossRef] [PubMed]
[7]. Rabinowitz MB, Kopple JD, Wetherill GW, Effect of food intake and fasting on gastrointestinal lead absorption in humansThe American Journal of Clinical Nutrition 1980 33(8):1784-88.10.1093/ajcn/33.8.17847405881 [Google Scholar] [CrossRef] [PubMed]
[8]. Ziegler EE, Edwards BB, Jensen RL, Mahaffey KR, Fomon SJ, Absorption and retention of lead by infantsPediatric Research 1978 12(1):29-33.10.1203/00006450-197801000-00008643372 [Google Scholar] [CrossRef] [PubMed]
[9]. Bogden JD, Gertner SB, Christakos S, Kemp FW, Yang Z, Katz SR, Chu C, Dietary calcium modifies concentrations of lead and other metals and renal calbindin in ratsThe Journal of Nutrition 1992 122(7):135110.1093/jn/122.7.13511619463 [Google Scholar] [CrossRef] [PubMed]
[10]. Mahaffey KR, Gartside PS, Glueck CJ, Blood lead levels and dietary calcium intake in 1-to 11-year-old children: The Second National Health and Nutrition Examination Survey, 1976 to 1980Pediatrics 1986 78(2):257-62. [Google Scholar]
[11]. Barltrop D, Meek F, Effect of particle size on lead absorption from the gutArchives of Environmental Health: An International Journal 1979 34(4):280-85.10.1080/00039896.1979.10667414475473 [Google Scholar] [CrossRef] [PubMed]
[12]. Aungst BJ, Fung HL, Kinetic characterization of in vitro lead transport across the rat small intestine: Mechanism of intestinal lead transportToxicology and Applied Pharmacology 1981 61(1):39-47.10.1016/0041-008X(81)90005-3 [Google Scholar] [CrossRef]
[13]. Bressler JP, Olivi L, Cheong JH, Kim Y, Bannona D, Divalent metal transporter 1 in lead and cadmium transportAnnals of the New York Academy of Sciences 2004 1012(1):142-52.10.1196/annals.1306.01115105261 [Google Scholar] [CrossRef] [PubMed]
[14]. Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM, Lead toxicity update. A brief reviewMedical Science Monitor 2005 11(10):329-36. [Google Scholar]
[15]. Al-Modhefer AJ, Bradbury MW, Simons TJ, Observations on the chemical nature of lead in human blood serumClinical Science 1991 81(6):823-29.10.1042/cs08108231662590 [Google Scholar] [CrossRef] [PubMed]
[16]. Rabinowitz MB, Toxicokinetics of bone leadEnvironmental Health Perspectives 1991 91:3310.1289/ehp.9191332040248 [Google Scholar] [CrossRef] [PubMed]
[17]. Roberts JR, Roberts J, Reigart JR, Ebeling M, Hulsey TC, Time required for blood lead levels to decline in nonchelated childrenJournal of Toxicology: Clinical Toxicology 2001 39(2):153-60.10.1081/CLT-10010383111407501 [Google Scholar] [CrossRef] [PubMed]
[18]. Rabinowitz MB, Wetherill GW, Kopple JD, Kinetic analysis of lead metabolism in healthy humansJ Clin Invest 1976 58:260-70.10.1172/JCI108467783195 [Google Scholar] [CrossRef] [PubMed]
[19]. Sanders T, Liu Y, Buchner V, Tchounwou PB, Neurotoxic effects and biomarkers of lead exposure: A reviewRev Environ Health 2009 24(1):15-45.10.1515/REVEH.2009.24.1.1519476290 [Google Scholar] [CrossRef] [PubMed]
[20]. Rabinowitz MB, Wetherill GW, Kopple of lead metabolism in healthy humansJournal of Clinical Investigation 1976 58(2):26010.1172/JCI108467783195 [Google Scholar] [CrossRef] [PubMed]
[21]. Bolanowska W, Distribution and excretion of triethyllead in ratsBr J Ind Med 1968 25:203-08.10.1136/oem.25.3.2035663424 [Google Scholar] [CrossRef] [PubMed]
[22]. Kimmel EC, Fish RH, Casida JE, Bioorganotin chemistry. Bioorganotin chemistry. Metabolism of organotin compounds in microsomal monooxygenase systems and in mammalsJ Agric Food Chem 1977 25(1):01-09.10.1021/jf60209a00212202 [Google Scholar] [CrossRef] [PubMed]
[23]. Omokhodion FO, Crockford GW, Lead in sweat and its relationship to salivary and urinary levels in normal healthy subjectsSci Total Environ 1991 103:113-22.10.1016/0048-9697(91)90137-4 [Google Scholar] [CrossRef]
[24]. Hohnadel DC, Sunderman FW Jr, Nechay MW, McNeely MD, Atomic absorption spectrometry of in microsomal monooxygenase systems and in mammalsJ Agric Food Chem 1977 25:01-09. [Google Scholar]
[25]. CDCCentre for disease control and prevention. Preventing lead poisoning in young children. A statement by the CDC 1991 Atlanta GAUS Dept. of Health and Human Services [Google Scholar]
[26]. Canfield RL, Henderson CRJ, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP, Intellectual impairment in children with blood lead concentrations below 10 microgram per deciliterN Engl J Med 2003 348:1517-26.10.1056/NEJMoa02284812700371 [Google Scholar] [CrossRef] [PubMed]
[27]. Chiodo LM, Jacobson SW, Jacobson JL, Neurodevelopmental effects of postnatal lead exposure at very low levelsNeurotoxicol Teratol 2004 26:359-71.10.1016/j.ntt.2004.01.01015113598 [Google Scholar] [CrossRef] [PubMed]
[28]. Flora SJ, Nutritional components modify metal absorption, toxic response and chelation therapyJournal of nutritional & environmental medicine 2002 12(1):53-67.10.1080/13590840220123361 [Google Scholar] [CrossRef]
[29]. Ercal N, Gurer-Orhan H, Aykin-Burns N, Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damageCurrent Topics in Medicinal Chemistry 2001 1(6):529-39.10.2174/156802601339483111895129 [Google Scholar] [CrossRef] [PubMed]
[30]. Saxena G, Flora SJ, Lead-induced oxidative stress and hematological alterations and their response to combined administration of calcium disodium EDTA with a thiol chelator in ratsJournal of Biochemical and Molecular Toxicology 2004 18(4):221-33.10.1002/jbt.2002715452883 [Google Scholar] [CrossRef] [PubMed]
[31]. Magos L, Webb M, Clarkson TW, The interactions of selenium with cadmium and mercuryCRC Critical Reviews in Toxicology 1980 8(1):110.3109/104084480090374907002474 [Google Scholar] [CrossRef] [PubMed]
[32]. Flora SJ, Metal poisoning: Threat and managementAl Ameen J Med Sci 2009 2(2):04-26. [Google Scholar]
[33]. Flora SJ, Pachauri V, Chelation in metal intoxicationInternational Journal of Environmental Research and Public Health 2010 7(7):2745-88.10.3390/ijerph707274520717537 [Google Scholar] [CrossRef] [PubMed]
[34]. Saalu LC, Ajayi GO, Adeneye AA, Imosemi IO, Osinubi AA, Ethanolic seed extract of grapefruit (Citrus paradisi Macfad)Int J Cancer Res 2009 5:44-52.10.3923/ijcr.2009.44.52 [Google Scholar] [CrossRef]
[35]. Suganthi V, Gowri S, Gurusamy K, Hepatoprotective activity of Cayratia carnosa on liver damage caused by lead acetate in ratsJ Nat Prod Plant Resour 2013 3(2):76-79. [Google Scholar]
[36]. Sharma V, Pandey D, Protective role of Tinospora cordifolia against lead-induced hepatotoxicityToxicology International 2010 17(1):1210.4103/0971-6580.6834321042467 [Google Scholar] [CrossRef] [PubMed]
[37]. Sharma VE, Verma RB, Sharma SH, Preliminary evaluation of the hepatic protection by pharmacological properties of the aqueous extract of Asparagus racemosus in lead loaded Swiss albino miceInt J Pharm Pharm Sci 2012 4(1):55-62.10.13005/bpj/267 [Google Scholar] [CrossRef]
[38]. Thenmozhi M, Dhanalakshmi M, Devi KM, Sushila K, Thenmozhi S, Evaluation of hepatoprotective activity of Leucas aspera hydroalcoholic leaf extract during exposure to lead acetate in male albino Wistar ratsAsian J Pharm Clin Res 2013 6(1):78-81. [Google Scholar]
[39]. Attia AM, Ibrahim FA, Nabil GM, Aziz SW, Antioxidant effects of ginger (Zingiber officinale Roscoe) against lead acetate-induced hepatotoxicity in ratsAfrican Journal of Pharmacy and Pharmacology 2013 7(20):1213-19.10.5897/AJPP2013.3465 [Google Scholar] [CrossRef]
[40]. Hemalatha K, Pugazhendy K, Jayachandran K, Jayanthi C, Meenambal M, Studies on the protective efficacy of Spirulina against lead acetate induced hepatotoxicity in Rattus norvegicusGroup 2012 2(3.17):0-15. [Google Scholar]
[41]. Kansal L, Sharma V, Sharma A, Lodi S, Sharma SH, Protective role of coriandrum sativum (coriander) extracts against lead nitrate induced oxidative stress and tissue damage in the liver and kidney in male miceInternational Journal of Applied Biology and Pharmaceutical Technology 2011 2(3):65-83. [Google Scholar]
[42]. Akilavalli N, Radhika J, Brindha P, Hepatoprotective activity of Ocimum sanctum Linn. Against lead induced toxicity in albino ratsAsian Journal of Pharmaceutical and Clinical Research 2011 4(2):84-87. [Google Scholar]
[43]. Abeer M, Grape seed extract (Vitisvinifera) alleviate neurotoxicity and hepatotoxicity induced by lead acetate in male albino ratsJournal of Behavioral and Brain Science 2012 2(1):12-19. [Google Scholar]
[44]. El-Ashmawy IM, Ashry KM, El-Nahas AF, Salama OM, Protection by turmeric and myrrh against liver oxidative damage and genotoxicity induced by lead acetate in miceBasic & Clinical Pharmacology & Toxicology 2006 98(1):32-37.10.1111/j.1742-7843.2006.pto_228.x16433888 [Google Scholar] [CrossRef] [PubMed]
[45]. Baxla SL, Gora RH, Kerketta P, Kumar N, Roy BK, Patra PH, Hepatoprotective effect of Curcuma longa against lead induced toxicity in Wistar ratsVet World 2013 6(9):664-67.10.14202/vetworld.2013.664-667 [Google Scholar] [CrossRef]
[46]. Fihri AF, Al-Waili NS, El-Haskoury R, Bakour M, Amarti A, Ansari MJ, Lyoussi B, Protective Effect of Morocco Carob Honey Against Lead-Induced Anemia and Hepato-Renal ToxicityCellular Physiology and Biochemistry 2016 39(1):115-22.10.1159/00044561027322825 [Google Scholar] [CrossRef] [PubMed]
[47]. Mehana EE, Meki AR, Fazili KM, Ameliorated effects of green tea extract on lead induced liver toxicity in ratsExperimental and Toxicologic Pathology 2012 64(4):291-95.10.1016/j.etp.2010.09.00120889321 [Google Scholar] [CrossRef] [PubMed]
[48]. Ghosh DE, Firdaus SB, Mitra EL, Dey MO, Chattopadhyay AI, Pattari SK, Dutta SA, Jana KU, Bandyopadhyay DE, Hepatoprotective activity of aqueous leaf extract of Murraya koenigii against lead-induced hepatotoxicity in male wistar ratInt J Pharm Pharm Sci 2013 5(1):285-95. [Google Scholar]
[49]. Velaga MK, Daughtry LK, Jones AC, Yallapragada PR, Rajanna S, Rajanna B, Attenuation of lead-induced oxidative stress in rat brain, liver, kidney and blood of male wistar rats by Moringa oleifera seed powderJournal of Environmental Pathology, Toxicology and Oncology 2014 33(4)10.1615/JEnvironPatholToxicolOncol.201401165625404379 [Google Scholar] [CrossRef] [PubMed]