Complex Arteriovenous Malformation in a Kidney with Multiple Renal Arteries: A Case Report
J Sanjay Prakash1, T Mathisekaran2, Nitesh Jain3, Pritam Chatterjee4, Sandeep Bafna5
1 DNB Urology Registrar, Department of Urology, Apollo Hospital, Chennai, Tamil Nadu, India.
2 DNB Urology Registrar, Department of Urology, Apollo Hospital, Chennai, Tamil Nadu, India.
3 Consultant Urologist, Department of Urology, Apollo Hospital, Chennai, Tamil Nadu, India.
4 Consultant Interventional Radiologist, Department of Radiology, Apollo Hospital, Chennai, Tamil Nadu, India.
5 Junior Consultant Urologist, Department of Urology, Apollo Hospital, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: J Sanjay Prakash, No. 7/21, Second Floor, Sri Labdhi Colony, TTK Road, Alwarpet, Chennai-600018, Tamil Nadu, India.
E-mail: sanprinarch@gmail.com
The abnormal communications between arteries and veins outside the capillary level are called Arteriovenous Malformation (AVM). A 25-year-old known hypertensive on irregular medications, presented with acute stroke (Computed Tomography (CT) brain revealed left putamen haemorrhage). On evaluation, all routine investigations were normal except for microscopic haematuria. Ultrasound (USG) KUB showed right AVM and on further evaluation with 320 slice Contrast Enhanced Computed Tomography (CECT) abdomen with angiogram and 3D reconstruction revealed right kidney supplied by three renal arteries. The second renal artery was dilated (1.1 cm diameter) and communicates directly with aneurysmally dilated right renal vein (2.9 cm diameter). Digital Subtraction Angiography (DSA) with angioembolisation was done four days’ postadmission. Large second renal artery with a direct Arteriovenous Fistula (AVF) draining into the renal vein with aneurysmal venous sacs was occluded with 16 mm, 12 mm and 10 mm coils. Complete obliteration of fistula was confirmed. Then the inferior most third renal artery was accessed and angiogram revealed RAVM with multiple feeders shunting into the venous sacs and it was occluded with 40% glue. Postoperative day one USG showed thrombosed venous aneurysmal sacs and occlusion of the arterial fistulae. He was discharged at four weeks with antihypertensive and antiseizure medications. During the follow-up there was no loss in the function of the kidney and micro or macroscopic haematuria was not detected.
Aneurysm, Arteriovenous fistula, Cirsoid, Digital subtraction angiography, Embolisation
Case Report
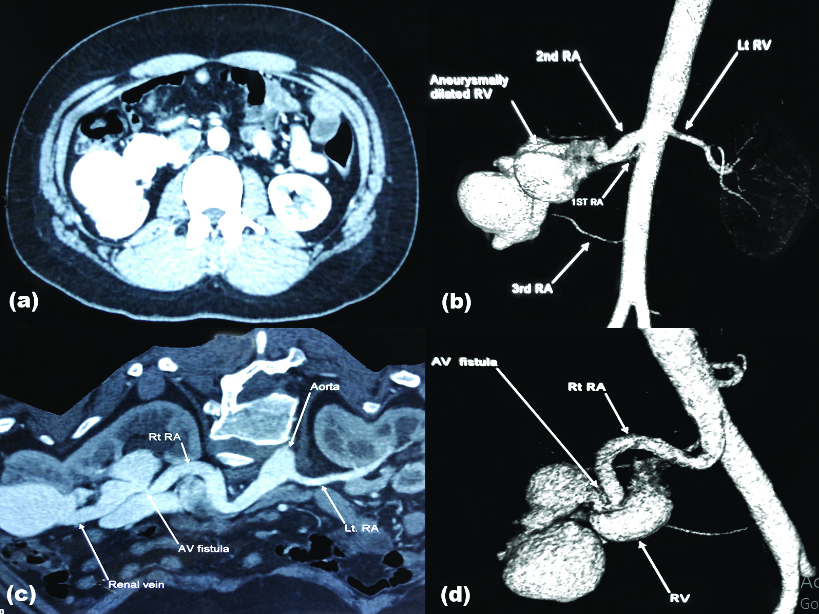
A 25-year-old male, known hypertensive on irregular medications, presented to the Emergency Department with sudden onset global headache, altered sensorium, right hemiparesis and right upper motor neuron facial palsy. On evaluation CT brain revealed left putamen haemorrhage. He was managed in multi-disciplinary critical care unit. All haematological investigations including renal function (creatinine 1 mg/dL) and coagulation profile (INR 1.1, APTT 29/31 sec, PT 14/13 sec) were within normal limits but urine microscopy revealed microscopic haematuria (6-8/HPF). ECHO cardiography was normal with ejection fraction of 66%. On routine screening abdominal USG, he was identified to have a Right Renal Arteriovenous Malformation (RAVM). Further evaluation with 320 slice CECT abdomen with angiogram and 3D reconstruction [Table/Fig-1] revealed right kidney supplied by three renal arteries. The second renal artery was dilated (1.1cm diameter) and communicates directly with aneurysmally dilated right renal vein (2.9 cm diameter). Rest of the study was normal. A 320 slice CECT of brain and angiography of neck revealed no evidence of vascular malformations.
(a) Contrast Enhanced Computed Tomography (CECT) abdomen, arterial phase showing immediate filling of the dilated draining vein; (b) reconstruction image revealing three arteries supplying the right kidney and an anuerysmally dilated renal vein; (c) and (d) Axial and reconstructed image revealing the site of Arteriovenous fistula respectively.
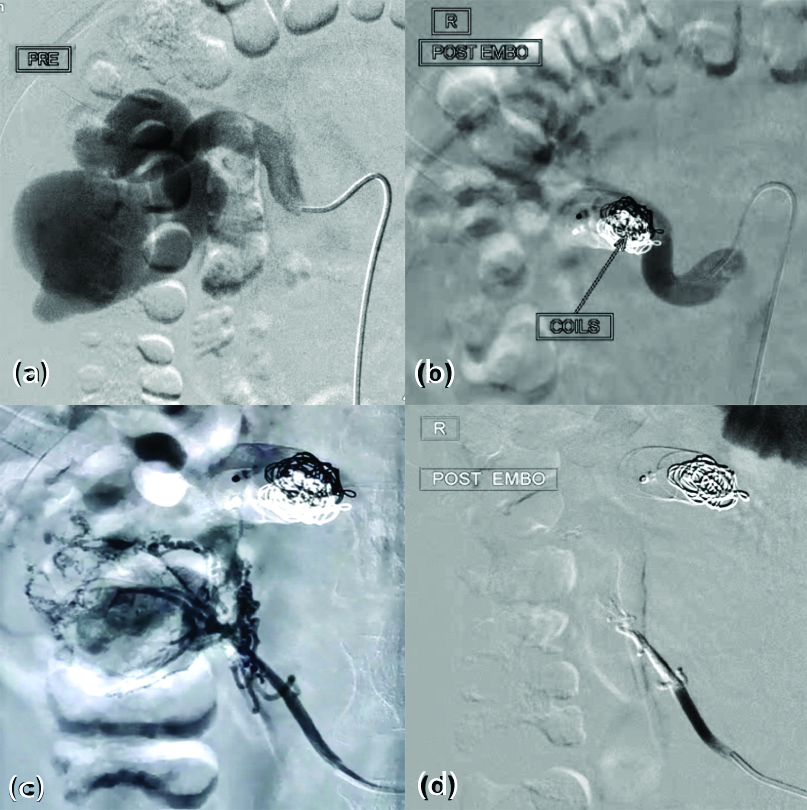
Multidisciplinary team involving vascular surgeon, urologist, nephrologist, interventional radiologist and multivisceral transplant surgeon discussed the management options with the family members. They were offered the options of observation, endovascular embolisation, nephrectomy and possible nephrectomy with backbench vascular reconstruction and auto-transplantation. They wished to undergo endovascular procedure. He was planned for Digital Subtraction Angiography (DSA) with angioembolisation after clearance from Cardiology and Neurology team four days’ post-admission. Under local anaesthesia with USG guidance, right Common Femoral Artery (CFA), left CFA and right Internal Jugular Vein (IJV) were accessed and 6Fr sheaths were inserted. Initial angiography revealed a large second renal artery with a direct Arteriovenous Fistula (AVF) draining into the renal vein with aneurysmal venous sacs. This AVF was occluded with 16 mm, 12 mm and 10 mm coils. Complete obliteration of fistula was confirmed. Then, the inferior most third renal artery was accessed and angiogram revealed RAVM with multiple feeders shunting into the venous sacs which were occluded with 40% glue [Table/Fig-2]. Complete obliteration was confirmed. First renal artery was accessed and angiogram confirmed major supply to the right kidney.
Digital Subtraction Angiography (DSA) images (a) large second renal artery directly communicating with anuerysmally dilated renal vein; (b) postcoil embolisation of the AVF, no contrast seen beyond the coils; (c) third artery with multiple feeders shunting into the venous sacs; (d) post-embolisation with 40% glue.
Postoperative Ultrasonography (USG) on day one revealed thrombosed venous aneurysmal sacs and occlusion of the arterial fistulae. Both intrarenal arterial and venous blood flow were normal. His creatinine was 1.1 mg/dL. His general condition gradually improved with regular physical rehabilitation and was discharged once neurologically fit after four weeks with antihypertensive and antiseizure medications. He was regularly followed-up in Neurology OPD with USG KUB, serum creatinine and urine microscopy. Dimercaptosuccinic Acid (DMSA) scan at three months revealed good parenchymal function with split renal function left 53% and right 47%.
Discussion
The abnormal communications between arteries and veins outside the capillary level are called AVMs [1]. RAVMs are abnormal communications between the intra-renal arterial and venous systems and are relatively rare (incidence-0.04%) [1]. RAVM are classified based on aetiology and angiographic features. Most common (70-75%) causes are trauma, inflammation, malignancy, iatrogenic where usually a single tortuously dilated artery communicates with a vein, in the form of an aneurysmal fistula (RAVF), congenital (14-27%) and idiopathic (2.8%) [2]. Angiographic features classify AVM as either cirsoid or aneurysmal type-The cirsoid type has a knotted, tortuous appearance comprising numerous vessels and multiple AV interconnections and the aneurysmal type is a direct RAVF without nidus-like components, which are often associated with aneurysmal dilatation of the feeding artery and/or draining veins [3]. In general, congenital lesions typically have a cirsoid appearance and acquired or idiopathic lesions are usually aneurysmal. RAVMs usually (75%) present as macro/microscopic haematuria or detected incidentally (asymptomatic) on imaging studies done for different reasons [4]. The other presentations are hypertension, headache, or congestive cardiac failure resulting from the large amount of blood flowing through the AVF [3]. The gold standard for the diagnosis and characterisation of RAVMs is DSA, it evaluates the lesion dynamically and delineates the feeder branches anatomically [5].
Authors presented a case of incidentally detected complex congenital RAVM (cirsoid type) with microscopic haematuria and angiography showing multiple arteries, second artery communicating with a large aneurysmal vein and also third artery having multiple feeders communicating with venous sacs (cirsoid type).
Treatment options available are surgical excision (such as partial nephrectomy, total nephrectomy, renal auto-transplantation, vessel ligation) or less invasive intravascular procedures [6]. In order to avoid nephrectomy, shorten hospital stay and reduce preoperative risk, trans-arterial embolisation may be regarded as the first-line treatment of RAVM [7]. The selection of the embolic material is based on the size and flow of the lesion and the type of RAVMs and the types of embolic materials include particles (gelatin sponge particles and polyvinyl alcohol particles), coils (pushable and detachable coils), vascular plugs, detachable balloons, and liquid materials [3]. Post-embolisation syndrome such as fever, loin pain, nausea, and vomiting may follow transcatheter arterial embolisation and can be minimised by selective embolisation of RAVMs which preserves the renal parenchyma [8]. There is also a risk of recanalisation or incomplete occlusion after incomplete or inadequate embolisation.
In a study by Jia ZY et al., they compared 12 cases of RAVM who underwent renal arterial embolisation. They claimed the existence of multiple fine feeding arteries arising from the proximal portion of different segmental renal arteries is the reason behind the incomplete embolisation and trying to embolise the multiple proximal feeders will cause a large area of renal parenchymal infarction, leading to loss of renal function and severe postoperative pain [9]. Our patient showed no signs of complications related to the procedure because in present case there was a functioning and patent first artery supplying the kidney hence, complete occlusion of second and third accessory arteries with coils and glue was possible without compromising the function of the kidney. During the follow-up, there was no loss in the function of the kidney and micro or macroscopic haematuria was not detected.
Conclusion(s)
Renal artery embolisation is an effective treatment for patients with renal AVM. Delineation of the feeding vessels by DSA is very important to effectively treat and prevent functional loss of the kidney. However, these facilities are not universally available. Timely management is essential to prevent any adverse outcomes.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 04, 2020
Manual Googling: Nov 05, 2020
iThenticate Software: Nov 27, 2020 (19%)
[1]. Cho KJ, Stanley JC, Non-neoplastic congenital and acquired renal arteriovenous malformations and fistulasRadiology 1978 129(2):333-43.10.1148/129.2.333704845 [Google Scholar] [CrossRef] [PubMed]
[2]. Osawa T, Watarai Y, Morita K, Kakizaki H, Nonomura K, Surgery for giant high-flow renal arteriovenous fistula: Experience in one institutionBJU Int 2006 97(4):794-98.10.1111/j.1464-410X.2006.06108.x16536776 [Google Scholar] [CrossRef] [PubMed]
[3]. Maruno M, Kiyosue H, Tanoue S, Hongo N, Matsumoto S, Mori H, Renal arteriovenous shunts: Clinical features, imaging appearance, and transcatheter embolisation based on angioarchitectureRadiographics 2016 36(2):580-95.10.1148/rg.201615012426871987 [Google Scholar] [CrossRef] [PubMed]
[4]. Tarif N, Mitwalli AH, Al Samayer SA, Abu-Aisha H, Memon NA, Sulaimani F, Congenital renal arteriovenous malformation presenting as severe hypertensionNephrol Dial Transplant 2002 17(2):291-94.10.1093/ndt/17.2.29111812884 [Google Scholar] [CrossRef] [PubMed]
[5]. Hatzidakis A, Rossi M, Mamoulakis C, Kehagias E, Orgera G, Krokidis M, Management of renal arteriovenous malformations: A pictorial review. Vol. 5Insights into Imaging 2014 Springer Verlag:523-30.10.1007/s13244-014-0342-424996396 [Google Scholar] [CrossRef] [PubMed]
[6]. Hermans B, Uvin P, Vanhoucke JL, Goeman L, Verhamme L, Boel K, EMJ European medical journal incidentally detected renal arteriovenous malformation: A case report and review of the literatureEMJ Urol 2017 [Google Scholar]
[7]. Carrafiello G, Laganà D, Peroni G, Mangini M, Fontana F, Mariani D, Gross hematuria caused by a congenital intrarenal arteriovenous malformation: A case reportJ Med Case Rep 2011 5(1):51010.1186/1752-1947-5-51021982481 [Google Scholar] [CrossRef] [PubMed]
[8]. Somani BK, Nabi G, Thorpe P, Hussey J, McClinton S, Therapeutic transarterial embolisation in the management of benign and malignant renal conditionsSurgeon 2006 4(6):348-52.10.1016/S1479-666X(06)80110-1 [Google Scholar] [CrossRef]
[9]. Jia ZY, Zhou CG, Xia JG, Zhao LB, Zhang W, Liu S, Endovascular treatment of 12 cases of renal arteriovenous malformations: The experience of 1 center and an overview of the literatureVasc Endovascular Surg 2018 52(1):46-51.10.1177/153857441774050929130853 [Google Scholar] [CrossRef] [PubMed]