Concurrent bacteremia in patients with dengue fever is rarely reported. Two and a half-year-old female child with fever, cough and cold for six days presented to Emergency Room (ER) with tachypnea, tachycardia and hepatomegaly. Investigations revealed dengue fever. Respiratory symptoms probed us to investigate the case further. High-Resolution Computed Tomography (HRCT) thorax showed moderate pleural effusion with collapse consolidation of left lung and a thin walled cavity with septations and fluid in left upper lobe. Child was treated with injection meropenem and vancomycin successfully.
Case Report
Two and a half-year-old female child was admitted in Paediatrics Department with complaints of fever for six days, cough and cold for five days. At admission her temperature was 101°F. She had productive cough with rhinorrhea. The child also had vomiting and loose stools-three episodes each, on the day of admission.
On examination child was pale, febrile, tachypnoeic with respiratory rate of 70/min, tachycardic, with heart rate of 150/min and Blood Pressure of 70/50 mmHg. Liver was palpable 3 cm below the right costal margin.
In Emergency Room (ER), she was started on oxygen at rate of 4 l/min, a bolus of normal saline at 20 mL/kg over 30 minutes and IV paracetamol.
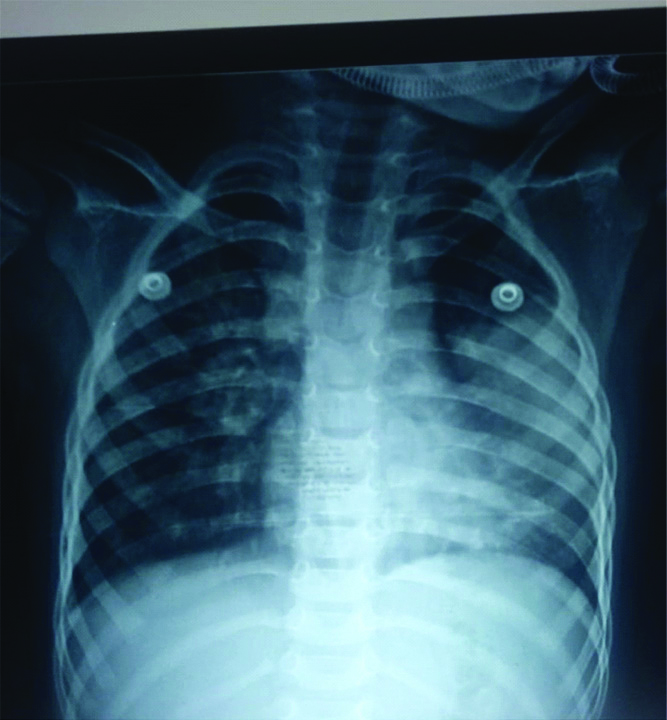
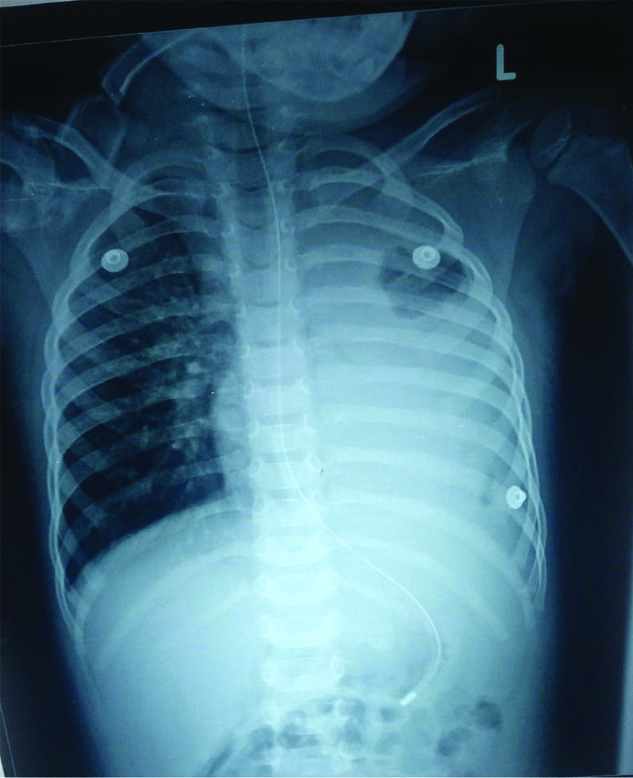
Initial investigations showed haemoglobin of 5.7 g/dL and PCV of 21.6%, for which packed red blood cell transfusion was done. Platelets count was 1.02 lacs/cu mm and total count was 3800 cells/cu mm. Chest X-ray showed consolidation of the left lung [Table/Fig-1]. In view of respiratory distress, child was started on ceftriaxone and oseltamavir for atypical pneumonia coverage. Dengue IgG and IgM were positive. Child was managed according to dengue protocol. On day five of admission child desaturated and repeat Chest X-Rays (CXR) was taken which showed evidence of collapse of left lung [Table/Fig-2]. She was started on oxygen through High-Flow Nasal Cannula (HFNC) and antibiotic changed to Piperacillin-Tazobacatm and Vancomycin. On day six, the child further deteriorated and was found to be in circulatory shock with weak peripheral pulses and firm hepatomegaly 7 cm under right costal margin. Shock was corrected with saline bolus.
ILL-defined homogenous opacity involving the left mid and lower zones- collapse/consolidation.
Left lung collapse with focal lucency in left upper zone.
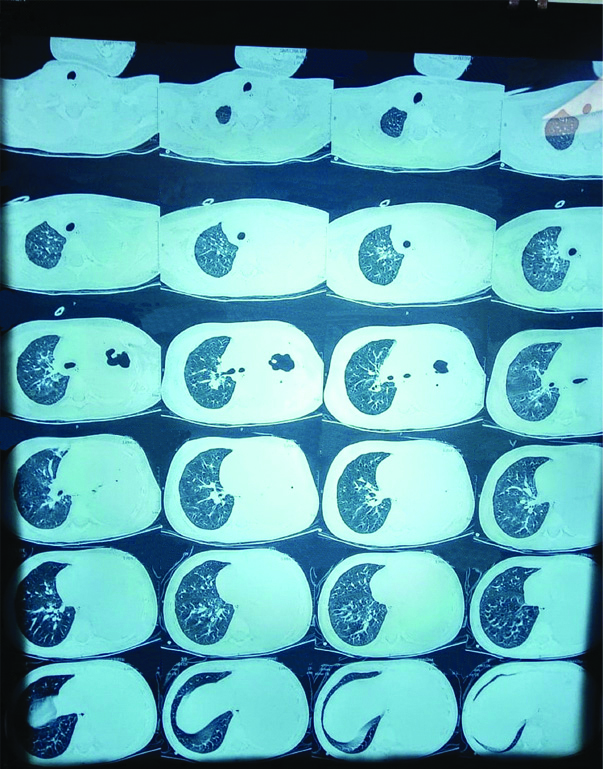
Her CXR showed no improvement and blood culture was sent and ultrasound abdomen done, which revealed minimal ascites. In view of poor clinical improvement antibiotic escalated to meropenem. As the child continued to be tachypneic with minimal air entry on the left lung fields, HRCT thorax was done on day seven, which showed moderate pleural effusion with collapse consolidation of left lung, mediastinal pull to left and a thin walled cavity in left upper lobe with septations [Table/Fig-3]. On day eight, Ultrasonography (USG) guided diagnostic pleural tap was done. Pleural fluid analysis revealed few inflammatory cells, Acid-Fast Bacilli (AFB) stain negative, gene expert for TB negative, no organism and no malignant cells.
HRCT thorax impression: moderate pleural effusion with collapse consolidation of left lung, with mediastinal pull to left.
Child was diagnosed with dengue shock with necrotising pneumonia and severe anaemia. Child was treated with meropenem and vancomycin for 14 days along with respiratory and circulatory support. Child improved and is on follow-up.
[Table/Fig-4] shows the sequential complete blood count values. It is noted that the White Blood Cell (WBC) count increased to levels suggestive of bacterial infection as the child recovered from dengue.
Shows the sequential complete blood count values. It is noted that the WBC count increased to levels suggestive of bacterial infection as the child recovered from dengue.
| CBC values | 24/10/2019 | 26/10 | 27/10 | 28/10 | 29/10 | 31/10 | 5/11/2019 |
|---|
| Hb (g/dL) | 5.7 | 8.6 | 8.5 | 9.7 | 8.5 | 8.5 | 7.8 |
| Platelet (lacs/cu mm) | 1.02 | 80000 | 1.20 | 1.48 | 1.32 | 3.17 | 6.29 |
| PCV (%) | 21.6 | 28.4 | 28.9 | 31.3 | 27.5 | 29 | 28.6 |
| TLC (cells/cu mm) | 3800 | 10700 | 9800 | 11300 | 9600 | 15700 | 16600 |
| MCV (fl) | 51.3 | 58.4 | 60 | 57.1 | 57.1 | 59.5 | 62.7 |
| MCH (pg) | 13.6 | 17.8 | 17.6 | 17.7 | 17.6 | 17.5 | |
| MCHC (g/dL) | 26.4 | 30.3 | 29.4 | 31.0 | 30.9 | 29.3 | 27.3 |
| DC P,L (%) | 63,30 | 77,19 | 78,17 | 65,28 | 59,32 | 63,27 | 66,25 |
CBC values #DC P,L: Differential count, Polymorphs and Lymphocytes
Discussion
Dengue fever is caused by virus belonging to the flavivirus family. There are four distinct serotypes of the virus that cause dengue fever (DENV-1, DENV-2, DENV-3 and DENV-4). It is transmitted by female mosquitoes of the species Aedes Aegypti. In 2015, official data from World Health Oranisation (WHO) member states reported more than 3.2 million dengue cases, including 10,200 cases of severe dengue and 1181 deaths. An estimated 2.5-3 billion people (approximately 40%-50% of the world’s population) are estimated to be at risk for dengue infection [1,2].
Dengue should be suspected when a high fever (40°C/104°F) is accompanied by two of the following symptoms during the febrile phase, severe headache, pain behind the eyes, muscle and joint pains, nausea, vomiting, rash. Warning signs include severe abdominal pain, persistent vomiting, rapid breathing, bleeding gums, fatigue, restlessness, blood in vomit.
For patients with suspected dengue virus disease, demonstration of presence of virus by rRT-PCR or NS1 antigen in serum sample within seven days of symptom is considered as laboratory confirmation of dengue. Enzyme-Linked Immunosorbent Assays (ELISA), may confirm the presence of a recent or past infection, with the detection of IgM and IgG anti-dengue antibodies. IgM antibodies are detectable at one week after infection and are highest at two to four weeks after the onset of illness and remain for about three months. The presence of IgM is indicative of a recent DENV infection and IgG is indicative of a past infection [3].
Children with dengue fever are categorised into three groups for management purposes. The first group is without warning signs, can be managed on an outpatient basis and asked to follow-up every 48 hours, to look for platelet count and haematocrit. The second groups of children are those with warning signs, needs admission to fever wards and treated with IV fluid replacement. The third groups of children are those with severe dengue, who needs to be admitted in intensive care units and treated with fluid replacement as well as treatment of complications [4]. Pulmonary complications are less common in dengue fever and can present as pleural effusion, pneumonitis, non-cardiogenic pulmonary oedema, acute respiratory distress syndrome, and pulmonary haemorrhage. Such complications coincide with capillary leak syndrome and thrombocytopenia. Diffuse alveolar haemorrhage is rare, and is typically related to severe and often fatal forms of the disease. Dengue viral antigens have been demonstrated in lung tissue in experimental infection and autopsy specimens, and the virus appears to potentially infect macrophages and lung endothelial and epithelial cells [5]. Haemoptysis has been reported in 1.4% of DENV infections. CT chest is the diagnostic test. The most common imaging findings in dengue are bilateral areas of ground-glass opacity or consolidation and bilateral pleural effusions [6].
Our case presented with fever, lower respiratory infection and haemodynamic instability. As there were many confirmed cases of dengue at the time of admission and patient had thrombocytopenia with leucopenia, dengue serology was sent and came out as Dengue IgM and IgG positive. Chest radiograph at admission showed consolidation, so we were inconclusive if pneumonia was a super-added infection or was a sequelae of dengue induced immunosuppression. Her pneumonia progressed to necrotising lesion with a prolonged course, despite starting antibiotics at admission. Throughout her stay at hospital her total count did not reflect the severity of the pneumonia causing a dilemma in identifying the causative agent. Children with dengue fever are considered recovered once the warning signs subside or haemodynamic instability improves. In our case, the child did not improve haemodynamically, even as the platelet and Packed Cell Volume (PCV) normalised, which gives us a diagnosis of probable bacterial infection (culture-negative), thus warranting the use of empirical antibiotics. We hypothesise that the lack of leucocytosis could be due to immunosuppression induced by dengue virus.
Ho LJ et al., and Chase AJ et al., in their study proposed that DENV infects and replicates in human dendritic cells and block Interferon (IFN)-α/β signaling in infected dendritic cells [7,8]. Antigen-presenting cells isolated from patients with acute DENV infection exhibit defects in T-cell priming.
Leukopenia, particularly affecting neutrophil and monocyte lineages, is well described and is thought to be related with bone marrow suppression [9,10]. Early blast cells are abortively infected, killed, and eliminated by phagocytosis by dendritic cells; infected adventitial reticular cells cause stromal failure of supporting haematopoiesis. Epithelial damage helps bacteria invade the tissue. Common bacteria that co-infect with dengue are Klebsiella, Staphylococcus, Pseudomonas, Salmonella and Leptospira. Lung epithelial cells are possible targets of Dengue virus, and Dengue virus infection induces apoptotic death of lung cells [11,12].
Dengue fever is typically a self-limiting disease. The patients who recover from dengue fever usually do not manifest any sequelae and develop immunity to the particular infecting serotype. The mortality rate of dengue fever is less than 1%. When treated, dengue haemorrhagic fever has a mortality rate of 2-5%. When left untreated, dengue haemorrhagic fever has a mortality rate as high as 50% [2].
Conclusion(s)
Dengue fever per se can cause immunosuppression due to damage of the dendritic cell and prevent T-cell priming. This immunosuppression can lead to secondary bacterial infection. One has to be vigilant in identifying other co-infections as the haemogram may not give adequate information due to bone marrow suppression.
CBC values
#DC P,L: Differential count, Polymorphs and Lymphocytes
[1]. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue [Google Scholar]
[2]. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, The global distribution and burden of dengueNature 2013 496(7446):504-07.10.1038/nature1206023563266 [Google Scholar] [CrossRef] [PubMed]
[3]. Dengue guidelines. CDC.gov.in/dengue [Google Scholar]
[4]. Rose W, Jacob JE, Adhikari DD, Verghese VP, Dengue illness in childrenCurr Med Issues 2017 15:95-105.10.4103/cmi.cmi_25_17 [Google Scholar] [CrossRef]
[5]. Jessie K, Fong MY, Devi S, Lam SK, Wong KT, Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and insitu hybridizationJ Infect Dis 2004 189(8):1411-18.10.1086/38304315073678 [Google Scholar] [CrossRef] [PubMed]
[6]. Marchiori E, Hochhegger B, Zanetti G, Pulmonary manifestations of dengueJ Bras Pneumol 2020 46(1):e2019024610.1590/1806-3713/e2019024632130344 [Google Scholar] [CrossRef] [PubMed]
[7]. Ho LJ, Hung LF, Weng CY, Wu WL, Chou P, Lin YL, Dengue virus type 2 antagonizes IFN-alpha but not IFN-gamma antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cellJ Immunol 2005 174:8163-72.10.4049/jimmunol.174.12.816315944325 [Google Scholar] [CrossRef] [PubMed]
[8]. Chase AJ, Medina FA, Muñoz-Jordán JL, Impairment of CD4+ T cell polarization by dengue virus-infected dendritic cellsJ Infect Dis 2011 203:1763-74.10.1093/infdis/jir19721606535 [Google Scholar] [CrossRef] [PubMed]
[9]. Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Early clinical and laboratory indicators of acute dengue illnessJ Infect Dis 1997 176:313-21.10.1086/5140479237695 [Google Scholar] [CrossRef] [PubMed]
[10]. La Russa VF, Innis BL, Mechanisms of dengue virus-induced bone marrow suppressionBaillieres Clin Haematol 1995 8:249-70.10.1016/S0950-3536(05)80240-9 [Google Scholar] [CrossRef]
[11]. Lee YR, Su CY, Chow NH, Lai WW, Lei HY, Chang CL, Dengue viruses can infect human primary lung epithelia as well as lung carcinoma cells, and can also induce the secretion of IL-6 and RANTESVirus Res 2007 126:216-25.10.1016/j.virusres.2007.03.00317416433 [Google Scholar] [CrossRef] [PubMed]
[12]. Hsu YL, Shi SF, Wu WL, Ho LJ, Lai JH, Protective roles of interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) in dengue virus infection of human lung epithelial cellsPLoS One 2013 8:e7951810.1371/journal.pone.007951824223959 [Google Scholar] [CrossRef] [PubMed]