Amiodarone is a frequently prescribed anti-arrhythmic drug which is used to treat ventricular and supraventricular tachyarrhythmia. Although it has excellent efficacy in controlling or preventing common arrhythmias, it is potentially associated with a variety of adverse effects, the most serious of these being pulmonary toxicity. Amiodarone-associated toxicities are usually seen in solid organs like lung, spleen and thyroid due to extension of its pharmacokinetic properties. The presentation is often subacute. Acute presentation with pleural involvement is distinctly uncommon in amiodarone toxicity and can pose diagnostic challenges. Here the case presented is of a 67 year old female with multiple co-morbidities on amiodarone therapy, who presented with massive pleural effusion and respiratory failure. Typical radiological findings along with exclusion of alternate causes with appropriate tests led to a diagnosis of amiodarone toxicity involving lung parenchyma, pleura, liver and other organs. She responded to withdrawal of drug, steroid therapy and supportive care.
Case Report
A 67-year-old female presented in Emergency Department with history of breathlessness, dry cough and lethargy of five days duration. Her past history was remarkable for carcinoma of left breast which was diagnosed 15 years back. She was managed successfully with modified radical mastectomy and chemoradiotherapy (5 Flurouracil, Adriamycin and cyclophosphamide-based regimen) 15 years back and remains under remission. She also gives history of hypothyroidism for last five years for which she is on thyroxine 125 mcg. She remains clinically euthyroid and periodic thyroid function tests have been within normal range. She had history of left middle cerebral artery infarct one year back (a cardio-embolic stroke secondary to new onset atrial fibrillation) for which she underwent tenectaplase thrombolysis one year back. She was initiated on amiodarone 600 mg per day since then for atrial fibrillation. She was under regular follow-up from Cardiology and Neurology Departments. She was seen in pulmonary medicine eight months back for evaluation of breathlessness. Then, she was evaluated with chest X-ray, Computed Tomography (CT) chest and spirometry which were with in normal limits.
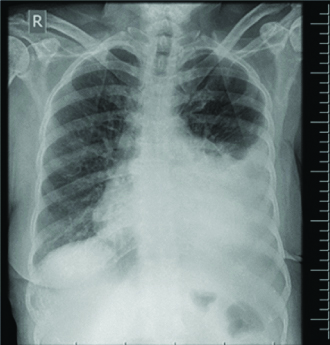
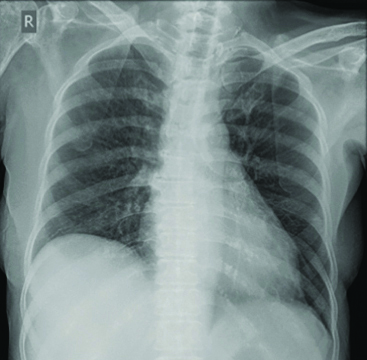
She denied any fever or expectoration at this visit. Initial neurological evaluation and neuroimaging revealed no new cerebrovascular events. She was tachypneic with respiratory rate of 28 per minute, blood pressure 100/70 mm of Hg, pulse rate was 110 per minute with irregular rhythm and her oxygen saturation is 93%. Electrocardiogram showed evidence of atrial fibrillation with a controlled ventricular rate and normal left ventricular function by 2D Echocardiography. Chest X-ray showed large left-sided pleural effusion [Table/Fig-1].
Initial chest X-ray showing moderate left-sided pleural effusion.
Considering the medical background, the possible differential diagnoses entertained included malignant effusion secondary to relapse of carcinoma breast, cardiac failure associated effusion, tuberculous pleural effusion, para pneumonic effusion, amiodarone toxicity etc. Her baseline blood reports are as follows: complete haemogram- Hb 12.4, Total count-13.6, Differential count P83, L9, C-reactive protein-61, Creatinine-0.76, Serum Glutamic-Oxaloacetic Transaminase (SGOT)- 83, Serum Glutamic-Pyruvic Transaminase (SGPT)-76, Trop I and serum Brain Natriuretic Peptide (BNP) levels were normal.
Therapeutic thoracentesis was done and 750 mL of straw coloured fluid was aspirated from left pleural space. Pleural fluid study revealed an exudative lymphocyte predominant fluid with low Adenosine Deaminase (ADA) values (TC 250; DC N 40 L60, Protein 3.8/LDH 282, ADA 10.1 IU). Pleural fluid culture returned sterile and mycobacterial tests were unrewarding. Pleural fluid cytology with cell block was negative for malignant cells.
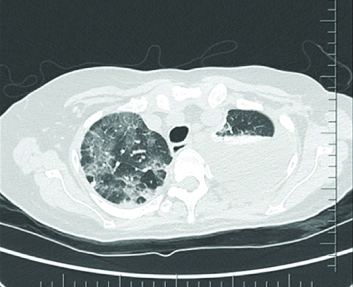
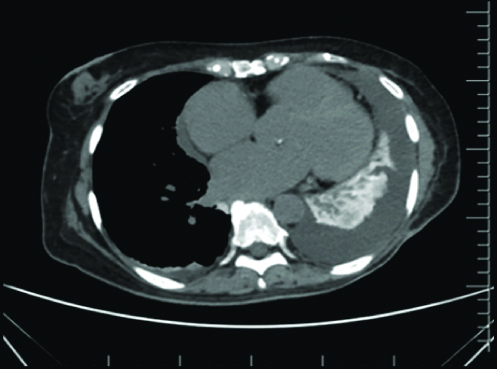
Further thoracic imaging with CT chest was undertaken [Table/Fig-2,3] which showed evidence of moderate left-sided and small right pleural effusion, ground glass changes and septal thickening involving multiple lobes, diffusely hyper-dense lung parenchyma, hyper-dense hepatic and splenic parenchyma as well as small bowel loops, tiny hyper-dense mesenteric and para-aortic lymph nodes. The overall picture, given the clinical background was consistent with amiodarone depositional disease with nonspecific interstitial pneumonia and drug-induced pleural effusion.
High-Resolution Computed Tomography (HRCT) Thorax showing evidence of interstitial thickening and pleural effusion.
Computed Tomography (CT) Chest mediastinal window with hyper-dense lung parenchyma.
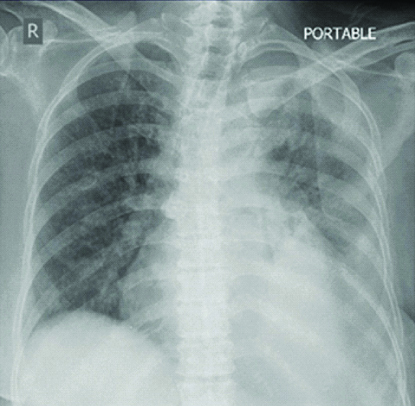
Correlating the clinical picture, CT findings and pleural fluid study reports, a diagnosis of amiodarone induced pleural effusion, drug-induced nonspecific interstitial pneumonitis and multiple organ deposition was made. The offender drug was discontinued and supportive care (supplemental oxygen, non-invasive ventilation and bronchodilators) was instituted. Systemic steroids (Methyl prednisolone 40 mg q 8h, followed by Prednisolone 60 mg per day) was initiated. She gradually improved with good clinical and radiological improvement [Table/Fig-4] and was discharged after three weeks on oral steroid 40 mg per day. Prednisolone was slowly tapered and was stopped over six months. Follow-up chest X-rays showed excellent radiological response with total resolution of pleural effusion and partial improvement of lung parenchymal shadows after six months [Table/Fig-5]. She continues to be under follow-up.
Chest X-ray at discharge showing significant resolution of effusion after three weeks.
Chest X-ray at six months of follow-up.
Discussion
Amiodarone is used for the prevention and treatment of a variety of tachyarrhythmias including life-threatening ventricular arrhythmias as well as supraventricular tachycardias such as atrial fibrillation [1]. It is a class three antiarrhythmic agent. It is a potassium channel blocker and its action results in increased action potential duration and a prolonged effective refractory period in cardiac myocytes. Myocyte excitability is decreased, preventing re-entry mechanisms and ectopic foci from perpetuating tachyarrhythmia. Usage of amiodarone has increased in the recent years in US and other countries. The popularity of amiodarone may be explained by its efficacy and usefulness in reducing or preventing common arrhythmias. However, adverse effects are not uncommon and organ toxicity may ensue. The chemical as well as pharmacokinetic properties of amiodarone are responsible for its side effects. The metabolite of this drug can accumulate in lungs, liver, heart, skin, corneal epithelium and peripheral nerves and causes local adverse events [2].
Amiodarone is unique with its pharmacokinetics and drug interactions. It’s two distinct properties, viz., high iodine content and high lipid solubility, are responsible for its untoward effects. The high iodine content is responsible for its effect on thyroid gland. Due to high lipid solubility, it accumulates extensively in adipose tissue and highly perfused organs such as the liver, lungs and spleen. The most common adverse event is related to micro-deposits in corneal and thyroid tissue, appearing in 85-100% of patients who receive chronic treatment. Pulmonary toxicity is the most dangerous complication and if unidentified is potentially life-threatening. The drug has got large volume of distribution which leads to delayed onset of action and a long elimination half-life as long as six months [3].
Amiodarone Pulmonary Toxicity (APT) is the most serious adverse effect and incidence varies from about 4 to 17% of patients. The incidence is higher in subjects with certain risk factors like higher cumulative dose and poor lung reserve. It is estimated that the incidence falls to 1.6- 2% in patients on 400 mg or lower doses against 5-15% in patients on more than 400 mg daily dosage [4]. But, cases of pulmonary toxicity were reported even with low doses too [5]. Some studies showed increased incidence in Japanese population even with low cumulative doses which points towards ethnic susceptibility [6]. APT is dependent on dose and duration of therapy [7]. But however, total cumulative dose is more important. The pulmonary toxicity is either due to direct drug-induced phospholipidosis, immune-mediated hypersensitivity [8] or an Angiotensin Converting Enzyme (ACE) related mechanism. The prevalence of pulmonary toxicity in amiodarone therapy was 2 to 17 percent [9]. Age more than 50 years male gender, amiodarone in high doses (>600 mg per day), duration of amiodarone therapy (more than six months), pre-existing lung disease, low diffusion capacity prior to treatment etc., are associated with higher lung toxicity. Interestingly, some studies showed high concentration of supplemental oxygen and mechanical ventilation to enhance pulmonary toxicity associated with amiodarone [10].
The pulmonary toxicity can occur at any time after commencement of treatment, the greater risk with individuals who receive daily dose of 400 mg or more for more than two months, or 200 mg daily more than two years [11].
The manifestation of pulmonary toxicity is variable and often mimics infection. Pulmonary toxicity can be asymptomatic, or manifest as acute or subacute presentation. There are case reports of pulmonary adverse effects which develop rapidly as following the first few days of amiodarone use, to chronically and insidiously over years [9]. An amiodarone lung effect similar to lipoid pneumonia has been described which is often asymptomatic. Acute presentations include acute hypersensitivity pneumonitis, Adult Respiratory Distress Syndrome (ARDS), eosinophilic pneumonia and diffuse alveolar haemorrhage. Subacute presentations include organising pneumonia, non-specific interstitial pneumonia-like and idiopathic pulmonary fibrosis-like interstitial pneumonia, lung nodules and mass lesions. There are case reports in literature where amiodarone toxicity were presented with interstitial pneumonia, nodular lesions and with or without fibrosis [12]. Pleural involvement is rare and in majority of cases it is associated with parenchymal involvement. Isolated effusions are extremely rare. There are case reports of isolated pleural effusions that too loculated effusion associated with amidarone [13]. Other rare presentations described in literature are unilateral pneumonitis [14], asymptomatic radiological infiltrates and conglomerate mass-like structure. In all these cases, diagnosis was made as diagnosis of exclusion and all of them improved with withdrawal of offender. Our lady had evidence of both parenchymal (in the form of Nonspecific Interstitial Pneumonia- NSIP) and pleural involvement.
Pathological changes occur either due to direct injury or T-cell mediated indirect lung injury. It causes free radical injury of cells and promote the accumulation of phospholipids in tissues. There will be type II pneumocytes hyperplasia and widening of alveolar interstitium with a cellular inflammatory infiltrate which leads to interstitial fibrosis [15].
APT is a diagnosis of exclusion. Clinically and radiologically, it mimics other common lung diseases like pulmonary infections, cardiac failure and other parenchymal diseases. Being a drug-induced lung disease, the diagnosis is usually made on clinical grounds with investigations helping to rule out alternate causes. Laboratory analyses do not help in establishing the diagnosis [16]. In our patient, drainage of pleural fluid with pleural fluid studies and other ancillary tests excluded alternate diagnoses and a CT scan of the chest and abdomen revealed findings typical of amiodarone toxicity. APT should be suspected in any patient taking amiodarone who has new or worsening symptoms and or new infiltrates on chest X-ray. In patient on amiodarone, a fall of Diffusing capacity of the Lungs for Carbon Monoxide (DLCO) by 15% to 20% may point towards a lung involvement [17]. Fibre-optic bronchoscopy with Broncho Alveolar Lavage (BAL) and trans-bronchial biopsy can be useful, especially in ruling out infections or other causes of diffuse lung disease. BAL fluid cytology may show evidence foamy macrophages with multilamellar intracytoplasmic bodies and lysosomes and loads of lipid material. Transbronchial lung biopsy shows different patterns like of Bronchiolitis Obliterans Organising Pneumonia (BOOP), Pleuroparenchymal fibroelastosis, non-specific interstitial pneumonias and diffuse alveolar damage [18,19]. Worsening cardiac failure and interstitial oedema may have to be ruled out by appropriate tests. Lung biopsy is rarely required. Improvement of disease on withdrawal of amiodarone aids in retrospective diagnosis [19].
Management depends on the clinical presentation. In emergency situations, life-saving measures to be initiated before establishing diagnosis.
First step in therapy is discontinuation of the agent. If the clinical manifestations are mild, usually the withdrawal alone of the drug leads to clinical and radiological improvement. If toxicity is of moderate severity, systemic steroids are advised though controversial. Most of the patients treated with corticosteroid showed reasonable improvement, but it is unclear whether it is due to antiinflammatory effect of steroid or due to the withdrawal of offending drug. There is a reported case of amiodarone pulmonary toxicity where the patient presented with pulmonary infiltrates which is resolved with corticosteroids despite continuing with amiodarone [14].
Prednisolone is usually used at a dose of 0.5/1 mg per kg of weight and for a long time, upto a year. In severe APT, high dose steroid and mechanical ventilation may be done. There are case reports of successful management of severe amiodarone-associated ARDS with haemoperfusion which is a process of removal of toxic substances with the help of an adsorbent [20]. Treatment response and prognosis are generally good unless not complicated with profound fibrosis and rapid progression to ARDS. Our patient responded very well to steroid therapy and replacement of offending drug. She has become totally asymptomatic with good clinical and radiological recovery in six months.
Prevention
There are no proven measures to prevent this. Some studies showed that concomitant administration of vitamin E has been shown to reduce the extent of pulmonary damage [21]. But early detection and prompt treatment can very well make the lungs normal. All patients initiated on amiodarone should be warned for adverse events. There are guidelines published by Goldschlager N et al., for clinicians prescribing amiodarone for early diagnosis and treatment of adverse events.
Practical guidelines for clinicians who treat patients with amiodarone [22]:
When an adverse effect is suspected, early clinical evaluation and investigations should be done so as to prevent progression to serious organ toxicity.
There should be baseline chest x-ray and pulmonary function test, including a DLCO.
Yearly chest X-ray may be obtained as long as patients are on amiodarone treatment.
An immediate chest X-ray and pulmonary function testing should be performed if there is clinical suspicion of pulmonary toxicity.
In order to detect extrapulmonary toxicity, thyroid function and liver enzymes to be monitored, as well as protective measures should be taken for skin photosensitivity.
Pulmonologist should be involved if abnormal baseline or follow-up chest radiography, Pulmonary function test value {particularly forced vital capacity and DLCO} or new onset cough or dyspnea.
Endocrinologist reference should be done if hyperthyroidism is suspected; the euthyroid sick syndrome should be specifically considered in acutely ill patients.
Electrophysiologist must be involved if worsening arrhythmia symptoms, until the arrhythmia problem stabilises, the patient may require intensified monitoring, electrophysiologic testing, ablative therapy, or pacemaker or Implantable Cardioverter Defibrillator (ICD) implantation, or even repeat defibrillation threshold testing.
Pregnant and paediatric patients who require amiodarone should be cautiously monitored.
Conclusion(s)
APT is an established entity. The early recognition is vital. Any new onset or worsening respiratory symptoms should be thoroughly evaluated to rule out APT. No definite dose or duration of treatment relationship is established. In case of any suspicion the drug should be stopped immediately and must evaluate thoroughly to rule out alternate causes.
[1]. Soar J, Perkins GD, Maconochie I, Böttiger BW, Deakin CD, Sandroni C, European Resuscitation Council Guidelines for Resuscitation: 2018 Update-Antiarrhythmic drugs for cardiac arrestResuscitation 2019 134:99-103.10.1016/j.resuscitation.2018.11.01830496838 [Google Scholar] [CrossRef] [PubMed]
[2]. Pollak PT, Clinical organ toxicity of antiarrhythmic compounds: Ocular and pulmonary manifestationsAm J Cardiol 1999 84(9A):37R-45R.10.1016/S0002-9149(99)00700-6 [Google Scholar] [CrossRef]
[3]. Vassallo P, Trohman RG, Prescribing amiodarone: An evidence-based review of clinical indicationsJAMA 2007 298(11):1312-22.10.1001/jama.298.11.131217878423 [Google Scholar] [CrossRef] [PubMed]
[4]. Sunderji R, Kanji Z, Gin K, Pulmonary effects of low dose amiodarone: A review of the risks and recommendations for surveillanceCan J Cardiol 2000 16(11):1435-40. [Google Scholar]
[5]. Ott MC, Khoor A, Leventhal JP, Paterick TE, Burger CD, Pulmonary toxicity in patients receiving low-dose amiodaroneChest 2003 123(2):646-51.10.1378/chest.123.2.64612576397 [Google Scholar] [CrossRef] [PubMed]
[6]. Yamada Y, Shiga T, Matsuda N, Hagiwara N, Kasanuki H, Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodaroneCirc J 2007 71(10):1610-16.10.1253/circj.71.161017895560 [Google Scholar] [CrossRef] [PubMed]
[7]. Jessurun GA, Crijns HJ, Amiodarone pulmonary toxicityBMJ 1997 314(7081):619-20.10.1136/bmj.314.7081.6199066469 [Google Scholar] [CrossRef] [PubMed]
[8]. Goldschlager N, Epstein AE, Naccarelli GV, Olshansky B, Singh B, Collard HR, A practical guide for clinicians who treat patients with amiodarone: 2007Heart Rhythm 2007 4(9):1250-59.10.1016/j.hrthm.2007.07.02017765636 [Google Scholar] [CrossRef] [PubMed]
[9]. PRIME PubMed | Amiodarone: Review of pulmonary effects and toxicity [Internet]. [cited 2020 Oct 23]. Available from: https://www.unboundmedicine.com/medline/citation/20553056/Amiodarone:_review_of_pulmonary_effects_and_toxicity_ [Google Scholar]
[10]. Saussine M, Colson P, Alauzen M, Mary H, Postoperative acute respiratory distress syndrome. A complication of amiodarone associated with 100 percent oxygen ventilationChest 1992 102(3):980-81.10.1378/chest.102.3.9801516446 [Google Scholar] [CrossRef] [PubMed]
[11]. Camus P, Martin WJ, Rosenow EC, Amiodarone pulmonary toxicityClin Chest Med 2004 25(1):65-75.10.1016/S0272-5231(03)00144-8 [Google Scholar] [CrossRef]
[12]. Sweidan AJ, Singh NK, Dang N, Lam V, Datta J, Amiodarone-induced pulmonary toxicity- A frequently missed complicationClin Med Insights Case Rep 2016 9:91-94.10.4137/CCRep.S3980927773995 [Google Scholar] [CrossRef] [PubMed]
[13]. Uong V, Nugent K, Alalawi R, Raj R, Amiodarone-induced loculated pleural effusion: Case report and review of the literaturePharmacotherapy 2010 30(2):21810.1592/phco.30.2.21820099996 [Google Scholar] [CrossRef] [PubMed]
[14]. McNeil KD, Firouz-Abadi A, Oliver W, Zimmerman PV, Amiodarone pulmonary toxicity-Three unusual manifestationsAust N Z J Med 1992 22(1):14-18.10.1111/j.1445-5994.1992.tb01702.x1580855 [Google Scholar] [CrossRef] [PubMed]
[15]. Martin WJ, Rosenow EC, Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part I)Chest 1988 93(5):1067-75.10.1378/chest.93.5.10673282816 [Google Scholar] [CrossRef] [PubMed]
[16]. Vasić NR, Milenković BA, Pešut DP, Stević RS, Jovanović DM, Drug induced lung disease-amiodarone in focusMedicinski Pregled 2014 67(9-10):334-37.10.2298/MPNS1410334V25546981 [Google Scholar] [CrossRef] [PubMed]
[17]. Gleadhill IC, Wise RA, Schonfeld SA, Scott PP, Guarnieri T, Levine JH, Serial lung function testing in patients treated with amiodarone: A prospective studyAm J Med 1989 86(1):04-10.10.1016/0002-9343(89)90221-0 [Google Scholar] [CrossRef]
[18]. Boras Z, Krizanac S, Rakusić N, Bronchiolitis obliterans with organising pneumonia in a patient treated with amiodaroneLijec Vjesn 2003 125(5-6):131-34. [Google Scholar]
[19]. Papiris S, Triantafillidou C, Kolilekas L, Markoulaki D, Manali E, Amiodarone review of pulmonary effects and toxicityDrug safety: An international Journal of Medical Toxicology and Drug Experience 2010 33:539-58.10.2165/11532320-000000000-0000020553056 [Google Scholar] [CrossRef] [PubMed]
[20]. Sato N, Kojima K, Horio Y, Goto E, Masunaga A, Ichiyasu H, Successful treatment of severe amiodarone pulmonary toxicity with polymyxin B-immobilized fiber column direct hemoperfusionChest 2013 143(4):1146-50.10.1378/chest.12-099423546489 [Google Scholar] [CrossRef] [PubMed]
[21]. Card JW, Racz WJ, Brien JF, Massey TE, Attenuation of amiodarone-induced pulmonary fibrosis by vitamin E is associated with suppression of transforming growth factor-beta1 gene expression but not prevention of mitochondrial dysfunctionJ Pharmacol Exp Ther 2003 304(1):277-83.10.1124/jpet.102.04320812490602 [Google Scholar] [CrossRef] [PubMed]
[22]. Goldschlager N, Epstein AE, Naccarelli G, Olshansky B, Singh B, Practical guidelines for clinicians who treat patients with amiodarone. Practice Guidelines Subcommittee, North American Society of Pacing and ElectrophysiologyArch Intern Med 2000 160(12):1741-48.10.1001/archinte.160.12.174110871966 [Google Scholar] [CrossRef] [PubMed]