Myroidesis a nonfermentative, gram-negative rod shaped bacterium which is an emerging multidrug resistant pathogen causing many serious hospital acquired infections like Catheter Associated Urinary Tract Infection (CAUTI). The authors report a case series (four cases) of CAUTI caused by Myroides species which was resistant to all tested antibiotics (ticarcillin-clavulanic acid, piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, cefoperazonesulbactam, imipenem, meropenem, amikacin, gentamycin ciprofloxacin, levofloxacin, colistin, tigecycline) and sensitive only to minocycline (minimum inhibitory concentration <1 μg/mL), in long-standing Diabetic Mellitus Type II patients. All the four patients were successfully treated with minocycline. Present cases highlight the importance of Myroides as a pathogen in Urinary Tract Infection (UTI) in diabetic patients, especially in nosocomial settings which clinicians should keep in mind.
Introduction
Myroides species are nonfermentative, gram-negative bacilli and is widely distributed in nature. It is an opportunistic pathogen which causes serious infections like septicaemia, pneumonia and UTI [1]. Published literature showed most of the Myroides infections occur in immunocompromised patients [2]. Management of infections caused by Myroides is challenging due to its high resistance to most antibiotics [2]. The global emergence and dissemination of multidrug resistant gram-negative superbugs, lead to the limited effectiveness of antibiotics for treating nosocomial infections [3,4]. The authors reported a case series (four cases) of CAUTI due to Myroides spp in diabetic patients admitted in Chhatrapati Shivaji Subharti Hospital, Meerut, Uttar Pradesh, India.
Case Series
Case 1
A 74-year-old male was admitted in unconscious state for six hours and was doing irrelevant talk over the past five days. The patient was taken to a local nursing home where he was diagnosed to have severe renal failure and referred to our hospital for further management and evaluation. The patient was a known case of Diabetes Mellitus (DM) Type II for the last nine years. The patient was taking tablet metformin 1000 mg twice a day but compliance was poor since last five months. Diagnostic Cerebrospinal Fluid (CSF) tap was done and microbiological and biochemical analysis was done. His creatinine was 3.0 mg/dL, random blood glucose level was 312 mg/dL, HbA1c was 9.5 mmol/mol and he had severe hyperkalemia with electrocardiograph changes. CSF was received in clinical microbiology laboratory for culture and sensitivity, but it was found to be bacteriologically sterile. He was initiated on haemodialysis for severe renal failure with hyperkalemia and urinary catheter was inserted. Also, Injection Insulin Subcutaneously (S/C), 100 units once a day was started.
Serology reports were negative for Hepatitis B surface antigen, Anti-HCV antibody and Anti-HIV antibody. The patient developed fever on fourth catheter day.
Case 2
A 58-year-old male patient with alleged history of road traffic accident was brought to the hospital within two hours of accident. The patient sustained head injury and underwent surgery in our hospital. The patient was put on mechanical ventilator and urinary catheterisation was done. The patient was a known diabetic for last 14 years. Random Blood Glucose level was 298 mg/dL and HbA1c was 9.1 mmol/mol. The patient was taking some oral hypoglycaemic drug from a local practitioner. Injection Colistin and Injection Piperacillin/Tazobactum was started. Injection Insulin S/C, 100 units twice daily was started.
Serology reports were negative for Hepatitis-B surface antigen, Anti-HCV antibody and Anti-HIV antibody. On fifth catheter day, the patient developed fever and had suprapubic tenderness.
Case 3
A 64-year-old female was admitted with history of cough, dyspnoea and chest pain for the past ten days. The patient was diagnosed with pneumonia in the local nursing home and referred to our hospital for further management and evaluation. The patient was a known case of DM for the last 15 years. The patient was taking tablet glimipride 1 mg once daily but was on Injection Insulin for last two years, with poor compliance since last 10 months. Diagnostic pleural tap was done and microbiological and biochemical analysis was done. Her random blood glucose level was 410 mg/dL, HbA1c was 11.5 mmol/mol. Pleural fluid was received in clinical microbiology laboratory for culture and sensitivity, but it was found to be bacteriologically sterile. Injection Meropenem was started. She was initiated on Inj. insulin 100 units once a day and urinary catheter was inserted.
The patient developed fever on third catheter day. Serology reports were negative for Hepatitis-B surface antigen, Anti-HCV antibody and Anti-HIV antibody. Urine sample was collected from the catheter port taking all aseptic precautions and sent to clinical microbiology laboratory for culture and sensitivity.
Case 4
A 70-year-old female was admitted with history of headache and blurring of vision for the two months and neck rigidity since past four days. The patient went to the local practitioner and referred to our hospital for further management and evaluation. The patient was a known case of DM for the last 25 years and was taking tablet metformin 2000 mg once daily which she took irregularly but had left the medication since last four months and started taking some preparation from a local practitioner. Diagnostic CSF tap was sent for microbiological and biochemical analysis. Her random blood glucose level was 320 mg/dL, HbA1c was 9.0 mmol/mol. Inj. Colistin Intravenously and Inj. Insulin S/C was started. CSF was received in clinical microbiology laboratory for culture and sensitivity, but it was found to be bacteriologically sterile. Urinary catheter was inserted. The patient developed fever on fourth catheter day.
Urine samples from all the four patients were processed in clinical microbiology laboratory and similar findings were observed. Gram stain was performed which revealed plenty of polymorphonuclear cells and gram-negative bacilli. It was inoculated on Cysteine Lactose Electrolyte Deficient (CLED) agar and incubated at 37°C. After overnight incubation, it showed growth of nonlactose fermenting colonies with yellow pigment [Table/Fig-1], fruity odour and significant bacteriuria (Colony Count ≥105 cfu/mL). Culture smear showed gram-negative bacilli [Table/Fig-2]. The isolate was oxidase positive, catalase positive and nonmotile. Biochemicals were inoculated and antibiotic sensitivity was done. On biochemical analysis, the isolate hydrolysed urea and showed as sacharolytic reaction in Hugh and leifson’s Oxidative and Fermentative Test [Table/Fig-3]. The isolate showed no reaction in the following biochemicals-Indole, Citrate, Triple sugar iron agar, glucose, lactose, sucrose, maltose, mannitol fermentation, nitrate, lysine, ornithine and arginine. It did not grow at 42°C. This pathogen was resistant to all antibiotics tested including Colistin and Polymyxin B and was found to be sensitive only to minocycline [Table/Fig-4].
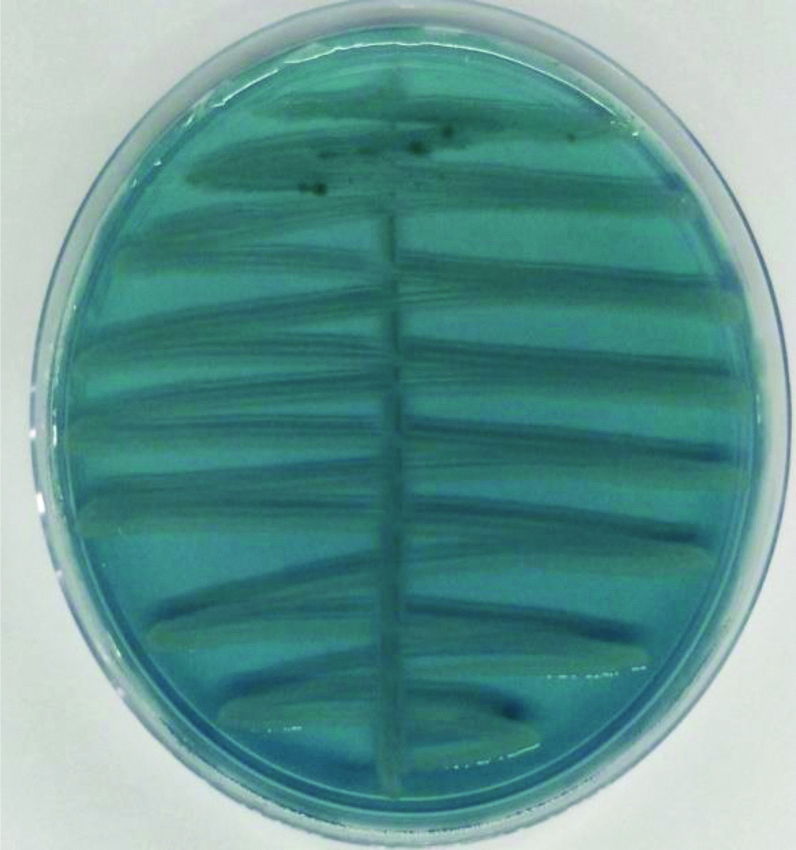
Nonlactose fermenting colonies on CLED agar.
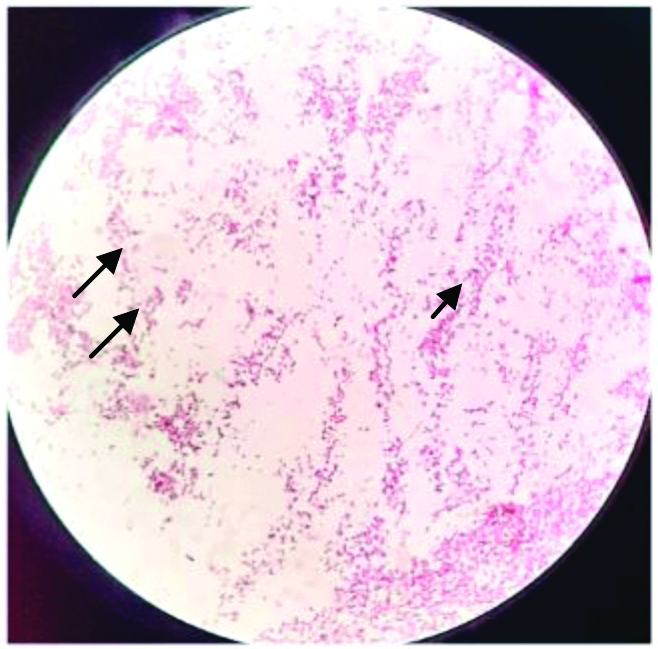
Culture smear showing gram-negative bacilli.
Biochemical reactions of Myroides species.
*I: Indole; U: Urease; C: Citrate; O/F: Hugh Leifson’so oxidation/fermentation media

Invitro susceptibility pattern of all the isolates of Myroides species.
| Antimicrobial agents | Isolate 1 | Isolate 2 | Isolate 3 | Isolate 4 |
|---|
| *MIC | #Int | *MIC | #Int | *MIC | #Int | *MIC | #Int |
|---|
| Ticarcillin/Clavulanic acid | ≥128/2 | R | ≥128/2 | R | ≥128/2 | R | ≥128/2 | R |
| Piperacillin/Clavulanic acid | ≥128/2 | R | ≥128/2 | R | ≥128/2 | R | ≥128/2 | R |
| Ceftazidime | ≥64 | R | ≥128 | R | ≥128 | R | ≥64 | R |
| Cefoperazone/Sulbactam | ≥64/4 | R | ≥64/4 | R | ≥64/4 | R | ≥128/16 | R |
| Cefepime | ≥64 | R | ≥64 | R | ≥64 | R | ≥64 | R |
| Aztreonam | ≥64 | R | ≥128 | R | ≥64 | R | ≥64 | R |
| Imipenem | ≥16 | R | ≥64 | R | ≥64 | R | ≥16 | R |
| Meropenem | ≥16 | R | ≥16 | R | ≥64 | R | ≥16 | R |
| Amikacin | ≥64 | R | ≥64 | R | ≥64 | R | ≥64 | R |
| Gentamycin | ≥64 | R | ≥128 | R | ≥64 | R | ≥64 | R |
| Ciprofloxacin | ≥4 | R | ≥4 | R | ≥8 | R | ≥4 | R |
| Levofloxacin | ≥8 | R | ≥8 | R | ≥8 | R | ≥8 | R |
| Minocycline | ≤1 | S | ≤1 | S | ≤1 | S | ≤1 | S |
| Tigecycline | ≥64 | R | ≥128 | R | ≥128 | R | ≥64 | R |
| Colistin | ≥16 | R | ≥16 | R | ≥16 | R | ≥16 | R |
*MIC: Minimum inhibitory concentration (μg/mL). #Int: Interpretation
The above finding was alarming and alerted us of growth of some unusual pathogen. On the basis of cultural characteristics and the extended biochemical reactions and intrinsic resistance to Colistin and Polymyxin-B the isolate was identified conventionally as Myroides species which was also reconfirmed by VITEK® 2 system (biomerieux, France) using GN REF 21341 card which also identified the isolate as Myroides Species. The drug susceptibility of the isolate was also performed using VITEK® 2 AST-N281 REF 414532 card. All the four cases have been summarised in [Table/Fig-5].
Summary of all the cases.
| Case 1 | Case 2 | Case 3 | Case 4 |
|---|
| Age (years)/Gender | 74/M | 58/M | 64/F | 70/F |
| Symptoms | Irrelevant talk and unconsciousness | Road traffic accident | Dry cough, dypnoea, chest pain | Headache, blurring of vision |
| Biochemical analysis | S. creatinine: 3.0 mg/dLRBS: 312 mg/dLHbA1c: 9.5 mmol/mol | RBS: 298 mg/dLHbA1c: 9.1 mmo/lmol | RBS: 410 mg/dLHbA1c: 11.5 mmol/mol | RBS: 320 mg/dLHbA1c: 9.0 mmol/mol |
| Microbiological analysis | Urine C/S: Growth of nonlactose fermenting gram-negative bacilli identified as Myroides Species sensitive only to Minocycline. | Urine C/S: Growth of nonlactose fermenting gram-negative bacilli identified as Myroides Species sensitive only to Minocycline. | Urine C/S: Growth of nonlactose fermenting gram-negative bacilli identified as Myroides Species sensitive only to Minocycline. | Urine C/S: Growth of nonlactose fermenting gram-negative bacilli identified as Myroides Species sensitive only to Minocycline. |
| Diagnosis | Acute kidney failure with Type II DM with CAUTI | Road Traffic Accident with Type II DM with CAUTI | Community acquired Pneumonia with Type II DM with CAUTI | Meningitis with Type II DM with CAUTI |
| Treatment | Injection Insulin S/C, 100 units once a dayHaemodialysisInj. Minocycline 200 mg Intravenous (IV) initially followed by 100 mg IV every 12 hours | Inj. Colistin 300 mg I/V every 12 hours and Inj. Piperacillin/Tazobactum 4 g I/V 8 hourly.Inj. Insulin S/C,100 units twice dailyInj. Minocycline 200 mg IV every 12 hours | Inj. Insulin 100 units once a dayInj. Meropenem 500 mg IV 8 hourlyInj. Minocycline 200 mg IV initially followed by 100 mg IV every 12 hours | Inj. Insulin100 units once a dayInj. Colistin300 mg I/V every 12 hoursInj. Minocycline 200 mg IV initially followed by 100 mg IV every 12 hours |
| Follow-up and outcome | Recovered and discharged after 28 days | Recovered and discharged after 40 days | Recovered and discharged after 19 days | Recovered and discharged after 22 days |
Speciation of Myroides cannot be done by Vitek; it is possible by Matrix Assisted Laser Desorption/Ionization-Time of Flight mass spectrometry (MALDI-TOF), but could not be done due to limited resources.
Discussion
The Myroides genus comprises of two species, Myroides Odoratus (former F. odoratum) and Myroides Odoratimimus, which are gram-negative rods, strictly aerobic, nonmotile, with yellow pigmentation and a characteristic fruity odour [5].
Myroides Odoratimimus is commonly found in the environment and frequently isolated from the immunocompromised patients. M. odoratus and M. odoratimimus primarily infects immunocompromised individuals [6]. UTI have been reported in patients with chronic nephritis, urinary retention, urinary calculi, and DM [7]. Nosocomial outbreaks of Myroides UTI have also been reported in the published literature [8]. Diabetes is one of the major risk factors associated with UTIs caused by Myroides. Likewise, all our patients were immunocompromised, long-standing diabetics.
The Antimicrobial Resistance (AMR) has reached alarming levels in different parts of the world. As a result, many available treatment options are becoming ineffective. The major concern in AMR is the dissemination of bacteria with resistance to several antibiotics, also known as “superbugs” like Myroides species [4].
The incidence of UTI caused by Myroides species is a rare phenomenon. The most important predisposing factor for hospital-acquired UTIs is urinary catheterisation, which reduces host defense mechanisms and offers easier access of germs to the bladder. In our cases, all the patients with Myroides UTI had an indwelling urinary catheter which is the most likely source of infection [9]. All were cases of CAUTI covered under surveillance by the infection control team of our hospital and informed to the treating clinician. The patients were kept under isolation with nursing barrier. Several authors have also reported similar cases of UTI in immunocompromised patients with indwelling catheters [1,4,10].
In literature, there are several cases which associate Myroides spp. with different types of infections such as soft tissue infections [11], sepsis [12], bacteremia [6], cellulitis [13], pericardial effusion [14], pediatric severe burn injury [15] and urosepsis [16].
Myroides spp. are known to be resistant to a wide range of antimicrobial agents, including betalactams, monobactams, carbapenems, and aminoglycosides [2]. Due to their multiple antibiotic resistance mechanisms, a fast and reliable identification method for Myroides spp. is needed [7]. Now-a-days, the range of community and hospital-acquired infections caused by atypical pathogens is continuously being updated. The emergence of these microorganisms is associated with and impacted on by infection control and antimicrobial stewardship.
All isolates reported in the present case series were extensive drug resistant strains, sensitive only to minocycline and resistant to all the other tested antimicrobials. Similar susceptibility pattern was noted by Licker M et al., in a case series in 2018, in which all the drugs were resistant except minocycline [18]. All the patients were started on Inj. minocycline. Follow-up was done and all responded well and survived. In the present case series, all the patients were immunocompromised having long-standing DM, suffered from CAUTI caused by myroides species and responded well to Minocycline.
Conclusion(s)
Clinicians should be aware of the ability of Myroides spp to cause UTI outbreaks as they are uncommon pathogens, especially in the immunocompromised population. Myroides should consider as a pathogen in CAUTI in diabetic patients especially in nosocomial settings. It is important to identify Myroides spp. infections rapidly with the help of automation in order to choose the best therapeutic regimen, considering the wide range of antibiotic resistance of these microorganisms. A well-designed antimicrobial stewardship associated with an efficient infection control is essential to limit the spread of these new emerging multidrug resistant pathogens.
[1]. Verma Y, Hemachander SS, Shah K, Urinary tract infection caused by Myroides spp. In diabetic patients: To be or not to beIndian J Case Reports 2018 4(1):01-03.10.32677/IJCR.2018.v04.i01.001 [Google Scholar] [CrossRef]
[2]. Holmes B, Snell JJ, Lapage SP, Flavobacteriumodoratum: A species resistant to a wide range of antimicrobial agentsJ Lin Pathol 1979 32:73-77.10.1136/jcp.32.1.73429582 [Google Scholar] [CrossRef] [PubMed]
[3]. Deris ZZ, The multidrug resistant gram negative superbugs threat require intelligent use of the last weaponMalays J Med Sci 2015 22(5):01-06. [Google Scholar]
[4]. Lorenzin G, Piccinelli G, Carlassara L, Scolari F, Caccuri F, Caruso A, Myroidesodoratimimus urinary tract infection in an immunocompromised patient: An emerging multidrug-resistant micro-organismAntimicrob Resist Infect Control 2018 7:96 [Google Scholar]
[5]. Vancanneyt M, Segers P, Torck U, Reclassification of Flavobacteriumodoratum (Stutzer 1929) strains to a new genus, Myroides, as Myroidesodoratuscomb. nov. and MyroidesodoratimimusspnovInt J Syst Bacteriol 1996 46(4):926-32.10.1099/00207713-46-4-926 [Google Scholar] [CrossRef]
[6]. Yazdani ETR, Dhiman N, Benavides R, Spak CV, Myroidesodoratimimus bacteremia in a diabetic patientProc Bayl Univ Med Cent 2015 28:342-43.10.1080/08998280.2015.1192926826130883 [Google Scholar] [CrossRef] [PubMed]
[7]. Hu SH, Yuan SX, Qu H, Jiang T, Zhou YJ, Wang MX, Antibiotic resistance mechanisms of MyroidesspJ Zhejiang Univ Sci B 2016 17:188-99.10.1631/jzus.B150006826984839 [Google Scholar] [CrossRef] [PubMed]
[8]. Yağci A, Cerikçioğlu N, Kaufmann ME, Malnick H, Söyletir G, Babacan F, Molecular typing of Myroidesodoratimimus (Flavobacteriumodoratum) urinary tract infections in a Turkish hospitalEur J Clin Microbiol Infect Dis 2000 19:731-32.10.1007/s10096007000111057514 [Google Scholar] [CrossRef] [PubMed]
[9]. Hooton TM, Bradley SF, Cardenas DD, Infectious Diseases Society of America. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of AmericaClin Infect Dis 2010 50(5):625-63.10.1086/65048220175247 [Google Scholar] [CrossRef] [PubMed]
[10]. Ktari S, Mnif B, Koubaa M, Mahjoubi F, Ben Jemaa M, Mhiri MN, Nosocomial outbreak of Myroidesodoratimimusurinary tract infection in a Tunisian hospitalJ Hosp Infect 2012 80:77-81.10.1016/j.jhin.2011.09.01022099498 [Google Scholar] [CrossRef] [PubMed]
[11]. Pompilio A, Galardi G, Gherardi G, Verginelli F, Geminiani C, Pilloni AP, Infection of recurrent calcaneal ulcer caused by a biofilm-producer Myroidesodoratimimus strainFolia Microbiol 2018 63:203-07.10.1007/s12223-017-0552-528956275 [Google Scholar] [CrossRef] [PubMed]
[12]. Benedetti P, Rassu M, Pavan G, Sefton A, Pellizzer G, Septic shock, pneumonia, and soft tissue infection due to Myroidesodoratimimus: Report of a case and review of Myroides infectionsInfection 2011 39:161-65.10.1007/s15010-010-0077-121246247 [Google Scholar] [CrossRef] [PubMed]
[13]. Bachmeyer C, Entressengle H, Khosrotehrani K, Goldman G, Delisle F, Arlet G, Cellulitis due to Myroidesodoratimimus in a patient with alcoholic cirrhosisClin Experim Dermatol 2007 33:97-98.10.1111/j.1365-2230.2007.02590.x18039344 [Google Scholar] [CrossRef] [PubMed]
[14]. Prateek S, Gupta P, Mittal G, Singh AK, Fatal case of pericardial effusion due to Myroidesodoratus: A rare case reportJ Clin Diagn Res 2015 9:DD01-02.10.7860/JCDR/2015/15120.674026672889 [Google Scholar] [CrossRef] [PubMed]
[15]. Soydan S, Ignak S, Demirei OU, Karadag G, Ocak Z, Myroides species in a Paediatric burn patientJ Clin Diagn Res 2017 11:DD03-04.10.7860/JCDR/2017/30021.10826 [Google Scholar] [CrossRef]
[16]. Ranjan M, Karade S, Rahi P, Singh SP, Sen S, Urosepsis due to multi drug resistant myroidesodoratimimus: A case reportInt J Curr Microbiol App Sci 2017 6:1930-35.10.20546/ijcmas.2017.608.228 [Google Scholar] [CrossRef]
[17]. Licker M, Sorescu T, Rus M, Cirlea N, Horhat F, Jurescu C, Extensively drug-resistant Myroidesodoratimimus-a case series of urinary tract infections in immunocompromised patientsInfect Drug Resist 2018 11:743-49.10.2147/IDR.S16106929849466 [Google Scholar] [CrossRef] [PubMed]