Giant Phyllodes Tumour in a Postmenopausal Woman: A Case Report
Kalaivani Vinayagam1, C Satish2, Abhishek Chaturvedi3
1 Associate Professor, Department of General Surgery, M S Ramaiah Medical College and Hospital, Bengaluru, Karnataka, India.
2 Senior Resident, Department of Surgical Oncology, M S Ramaiah Medical College and Hospital, Bengaluru, Karnataka, India.
3 Junior Resident, Department of General Surgery, M S Ramaiah Medical College and Hospital, Bengaluru, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kalaivani Vinayagam, #86, 4th Cross, C Sector, Amruth Nagar, Bangalore-560092, Karnataka, India.
E-mail: dr.vani_rajan@yahoo.com
Phyllodes Tumours (PT) of the breast are uncommon fibroepithelial lesions accounting for less than 1% of breast tumours. These tumours commonly occur in females during the fourth and fifth decade of life. They have a morphological resemblance to the intra-canalicular fibroadenoma. Their median size is around 4 cm, however if the size is more than 10 cm it’s called a giant phyllodes tumour accounting to less than 10% of phyllodes tumours. Clinically and histologically, they are difficult to be differentiated from fibroadenoma and a wide local excision is the mainstay of treatment. Hereby, Authors are reporting a case of giant phyllodes tumour in a 56-year-old post-menopausal female patient. She presented with a lump of 27×20 cm of two years duration occupying the entire left breast. Since, the lump had rapidly increased in size in the last six months of presentation, a clinical diagnosis of giant phyllodes tumour with malignant transformation was the provisional diagnosis. She underwent simple mastectomy with split-thickness skin graft. The final histopathology was reported as borderline phyllodes tumour and the patient was on a regular follow-up since the last 10 months.
Breast neoplasm, Cystosarcoma, Fibroepithelial lesions, Spindle cell
Case Report
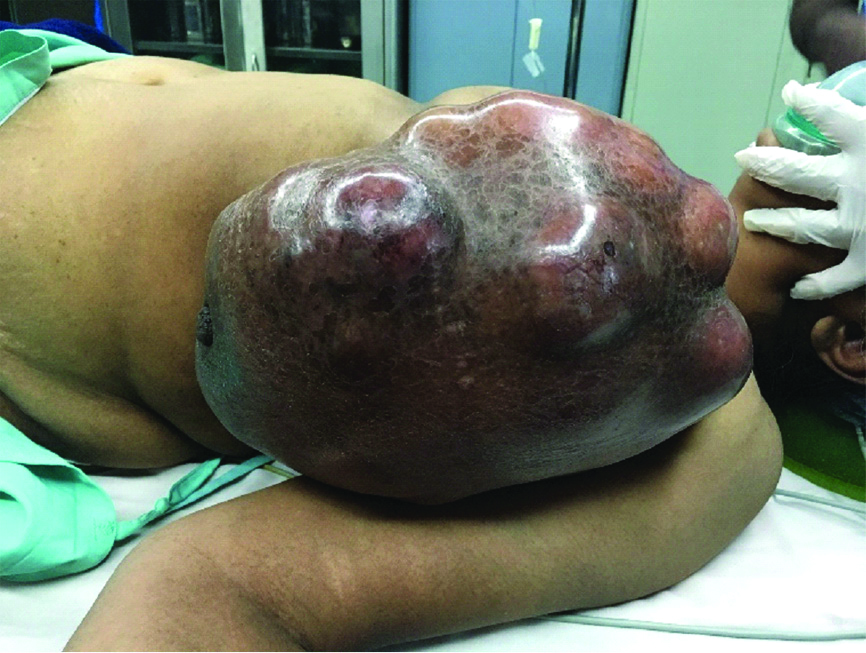
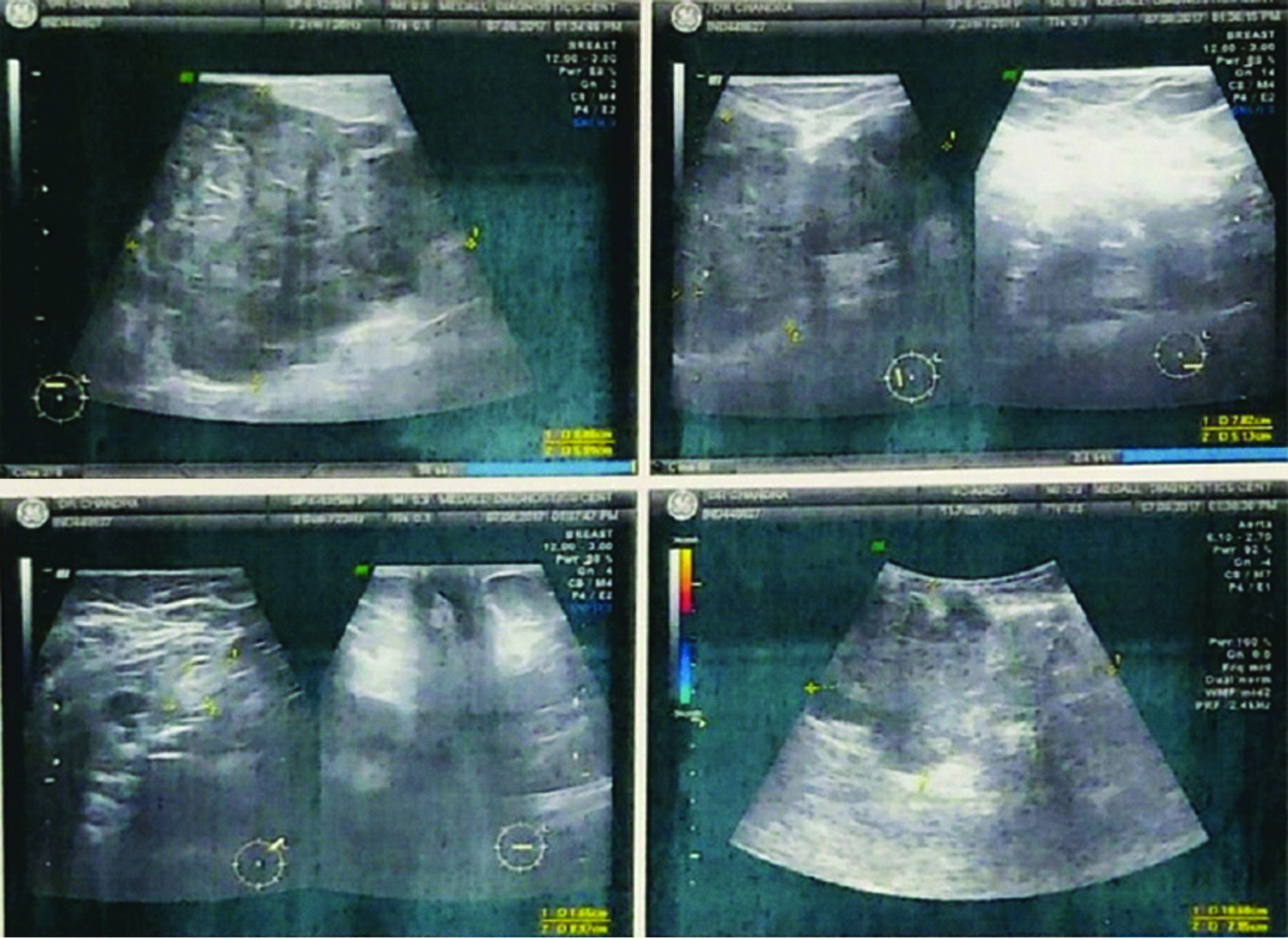
A 56-year-old female patient presented to the Out Patient Department with a lump in the left breast of two years duration which was slowly increasing in size. However, the lump increased in size rapidly, associated with dragging pain in the last six months affecting her day-to-day activities. She had no associated co-morbidities or history of hospitalisation in the past. On examination, there was a huge lump of 27×20 cm involving the entire breast, with a bosselated surface and variable consistency. The skin over the swelling was shiny, stretched and fixed at few places. It was not fixed to the underlying muscles, with no axillary lymph nodes palpable. [Table/Fig-1]. A clinical diagnosis of phyllodes tumour with probable malignant transformation was made in view of her history and clinical findings; however, sarcoma was also considered a possibility. Routine blood investigations were within normal range, chest X-ray and ultrasound abdomen was normal. Sonomammography revealed a lobulated hypoechoic large lesion, 20.6×17 cm in upper inner quadrant extending to lower quadrant and towards nipple of left breast [Table/Fig-2]. Trucut biopsy showed linear tissue cores showing proliferating spindle cells in fascicles, spindled nuclei and bipolar cytoplasmic processes in cells, no significant nuclear atypia, no mitosis and myxomatous stroma. No lobular or ductal structures. She underwent simple mastectomy and the defect was covered with split-thickness skin graft. The tumour was weighing 4.8 kg and the postoperative period was uneventful.
Preoperative clinical photograph.
Showing left breast PT with bosselated surface with stretched and shiny skin.
Sonomammography showing lobulated hypoechoic large lesion 20.6×17 cm in upper inner quadrant, extending to lower quadrant and towards nipple of left breast.
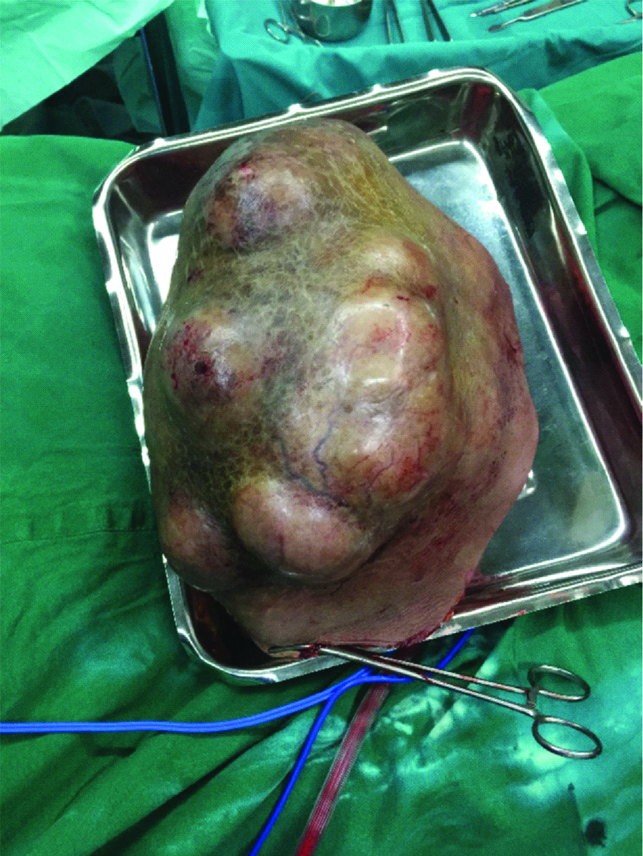
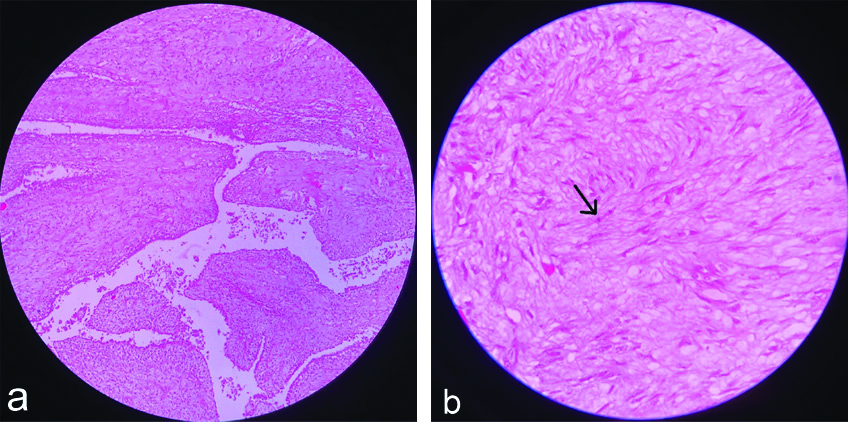
The specimen of breast was measuring 27×18×12 cm [Table/Fig-3] and the histopathology was reported as marked proliferation of stromal cells, which were cellular with spindle-shaped nuclei showing mild nuclear pleomorphism with mitotic count of 6-8 high power field (hpf) and an extensive area of hyaline degeneration was seen. Final impression was borderline phyllodes tumour of the breast and the surgical margin was free from tumour [Table/Fig-4a,b]. Patient is on a regular follow-up for the last 10 months and has not received any treatment post-surgery.
Excised breast tumour measuring 27×18×12 cm showing bosselated surface, stretched and shiny skin with visible dilated veins.
a) Histopathological image showing Leaf like pattern with epithelial and stromal components. (X100 magnification, haematoxylin and eosin stain); b) Histopathological image showing spindle-shaped stromal cells proliferation and mitosis. (X400 magnification, Haematoxylin and Eosin stain).
Discussion
The term “phyllodes” means leaf-like and refers to the papillary projections [1]. Most of the tumours occur in women of a median age of 42-45 years and approximately 10-15 years older than the median age for fibro adenomas of the breast [2]. PTs accounts to less than 1% of all breast tumours and has an incidence of 2.1 per million with a median size of about 4 cm [3]. A giant PT is more than 10 cm and they account to less than 10%. Giant PT of size of 50×50 cm has been reported in the literature [2]. They have a morphological resemblance to the intra-canalicular fibroadenoma at the benign end of the spectrum and at the other end of the spectrum; the malignant PT may be mistaken for primary breast sarcoma or spindle cell metaplastic carcinoma [4]. Clinically and histologically, they are difficult to be differentiated from fibro adenoma and a wide local excision is the mainstay of treatment. In 1981 the World Health Organisation (WHO) adopted the term tumour and are sub classified as benign, borderline and malignant based on the degree of stromal cellular atypia, permeative margins and mitotic activity of at least 10/10 hpf [1].
There are no pathognomonic mammographic or ultrasound features of PT. Diagnosis is established through core needle biopsy which has a sensitivity of 99% and negative predictive value and positive predictive value 93% and 83%, respectively [5] and a 75% sensitivity in differentiating from fibro adenoma. Stromal overgrowth was more often seen in specimens from phyllodes tumour as compared to fibro adenomas [6]. In this patient true-cut biopsy was reported as benign phyllodes tumour however the histopathological diagnosis was borderline PT. Less than 1% have pathological lymph nodes.
The mainstay of treatment of PT tumour is surgical, wide excision with adequate margins, a surgical margin of 1 cm is accepted [1]. If the tumour involves the entire breast tissue as in this patient, a simple mastectomy is advocated. A routine axillary dissection is not recommended as less than 1% have pathological lymph nodes. Though malignancy was suspected in this patient, she had no clinical or radiological enlargement of lymph nodes and a simple mastectomy was done. As the tumour was occupying the entire breast with skin involvement there was a wide defect requiring split-thickness skin graft.
A close follow-up with frequent breast examinations and imaging tests is recommended after surgery. They have a high recurrence rate of 10-40% [1]. Recurrence is related to the histological grade (33% for malignant tumour and 10% for benign). If the size of the tumour is more than 10 cm, the prevalence of local recurrence is four times greater than smaller tumours, if surgical margin is less than 1 cm, there is an increase in the risk by five times and presence of stromal overgrowth increased risk by seven times [7]. Malignant potential though very rare, lungs are the most common metastatic site followed by the skeleton, heart and liver [8]. Malignant PT has haematogenous metastasis in 22% of cases [9]. Metastasis may appear without local recurrence. According to multivariate analysis, young age (<35 years), tumour necrosis and positive surgical margins were the prognostic factors [10]. Young age was a favorable prognostic factor in most of the studies.
Multiple studies have shown the use of postoperative adjuvant radiation therapy is useful in reducing rates of local recurrence [11]. Few other studies have shown that adjuvant chemotherapy and radiotherapy have not been proven to be useful in the treatment of PT.
Conclusion(s)
Giant phyllodes tumours of the breast are a rare cause for rapidly enlarging painless lumps in middle aged women. A core needle biopsy is needed for diagnosis and has to be differentiated from fibroadenomas. However, a definitive diagnosis is possible only after excision. Surgical treatment though is the mainstay of treatment, depends on the clinical and histological diagnosis as the extent of surgery is very important in terms of further recurrence. The patient should be counselled for the need of surgery, possibility of malignancy and the need for adjuvant treatment if necessary and most importantly the need for regular follow-up.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jun 30, 2020
Manual Googling: Aug 28, 2020
iThenticate Software: Nov 25, 2020 (22%)
[1]. Calhoun K, Lawton TJ, Kim JM, Lehman CD, Anderson BO, Phyllodes tumoursDiseases of the breast. Harris J, Lippman ME, Osborne CK, Morrow M (eds) 2010 Lippincott Williams and Wilkins:781-92. [Google Scholar]
[2]. Karim RZ, Gerega SK, Yang YH, Spillane A, Carmalt H, Scolyer RA, Phyllodes tumours of the breast: A clinicopathological analysis of 65 cases from a single institutionBreast 2009 18(3):165-70.10.1016/j.breast.2009.03.00119329316 [Google Scholar] [CrossRef] [PubMed]
[3]. Albalawi IA, A huge phyllodes tumour in the breast: A case reportElectron Physician 2018 10(6):695110.19082/695130034663 [Google Scholar] [CrossRef] [PubMed]
[4]. Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, Brogi E, Phyllodes tumours of the breast: a consensus reviewHistopathology 2016 68(1):05-21.10.1111/his.1287626768026 [Google Scholar] [CrossRef] [PubMed]
[5]. Komenaka IK, El-Tamer M, Pile-Spellman E, Hibshoosh H, Core needle biopsy as a diagnostic tool to differentiate phyllodes tumour from fibroadenomaArch surgery 2003 138(9):987-90.10.1001/archsurg.138.9.98712963656 [Google Scholar] [CrossRef] [PubMed]
[6]. Lee AH, Hodi Z, Ellis IO, Elston CW, Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breastHistopathology 2007 51(3):336-44.10.1111/j.1365-2559.2007.02786.x17727475 [Google Scholar] [CrossRef] [PubMed]
[7]. Zissis C, Apostolikas N, Konstantinidou A, Griniatsos J, Vassilopoulos PP, The extent of surgery and prognosis of patients with phyllodes tumour of the breastBreast cancer Res Treat 1998 48(3):205-10.10.1023/A:10059808313599598867 [Google Scholar] [CrossRef] [PubMed]
[8]. Abe M, Miyata S, Nishimura S, Iijima K, Makita M, Akiyama F, Malignant transformation of breast fibroadenoma to malignant phyllodes tumour: Long-term outcome of 36 malignant phyllodes tumoursBreast Cancer 2011 18(4):268-72.10.1007/s12282-009-0185-x22121516 [Google Scholar] [CrossRef] [PubMed]
[9]. Walravens C, De Greef C, Giant phyllodes tumour of the breastJ Plast, Reconstr & Aesthet Surg 2008 61(10):e9-11.10.1016/j.bjps.2007.10.01818042445 [Google Scholar] [CrossRef] [PubMed]
[10]. Spitaleri G, Toesca A, Botteri E, Bottiglieri L, Rotmensz N, Boselli S, Breast phyllodes tumour: A review of literature and a single center retrospective series analysisCrit Rev Oncol Hematol 2013 88(2):427-36.10.1016/j.critrevonc.2013.06.00523871531 [Google Scholar] [CrossRef] [PubMed]
[11]. Barth RJ, Borderline and malignant phyllodes tumours: How often do they locally recur and is there anything we can do about it?Ann Surg Oncol 2019 26(7):1973-75.10.1245/s10434-019-07278-y30863938 [Google Scholar] [CrossRef] [PubMed]