An Unusual Presentation of Leiomyosarcoma Posing a Diagnostic Dilemma: A Case Report
Smita Singh1, Kusha Sharma2, Vipan Kumar3, Partap Yadav4
1 Associate Professor, Department of Pathology, Lady Hardinge Medical College, New Delhi, India.
2 Senior Resident, Department of Pathology, Lady Hardinge Medical College, New Delhi, India.
3 Senior Resident, Department of Paediatric Surgery, SSKH and KSCH, New Delhi, India.
4 Associate Professor, Department of Paediatric Surgery, SSKH and KSCH, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Kusha Sharma, C-2/2001, Vasant Kunj, New Delhi, India.
E-mail: drkushasharma@gmail.com
Leiomyosarcoma (LMS) is a malignant tumour of smooth muscle origin commonly seen in genital and gastrointestinal location. However, its presence in the head and neck region in a young child is extremely rare. Authors present a unique case of LMS in a one year and five-month-old child who presented with a painless mass in the right temporal region of the head. Histopathological examination revealed a highly cellular tumour arranged in intersecting fascicles of spindle cells with brisk mitotic activity and interspersed areas of necrosis. On Immunohistochemistry (IHC), the tumour cells were positive for Smooth Muscle Actin (SMA), Desmin and Vimentin and negative for S100 and Myogenin. A diagnosis of LMS was thus, made. Head and neck sarcoma is a broad entity encompassing plethora of differentials with closely overlapping morphological features which renders them diagnostically challenging, this can be resolved by employing various immunohistochemical stains readily available in all laboratories. This case highlights the combined role played by histopathology and immunohistochemistry in arriving at the correct diagnosis. To the best of our knowledge, this is the first case of LMS reported in the temporal region of head and is distinct with respect to its rare incidence, location and age at presentation. LMS at this site may masquerade as deceptively benign painless mass and may not be suspected initially, however one should bear in mind that these are moderate-to-high grade tumours and any delay in management may portend poor prognosis. Timely and aggressive surgical management is thus, the mainstay of treatment and critical to patient survival.
Child, Mass, Temporal region
Case Report
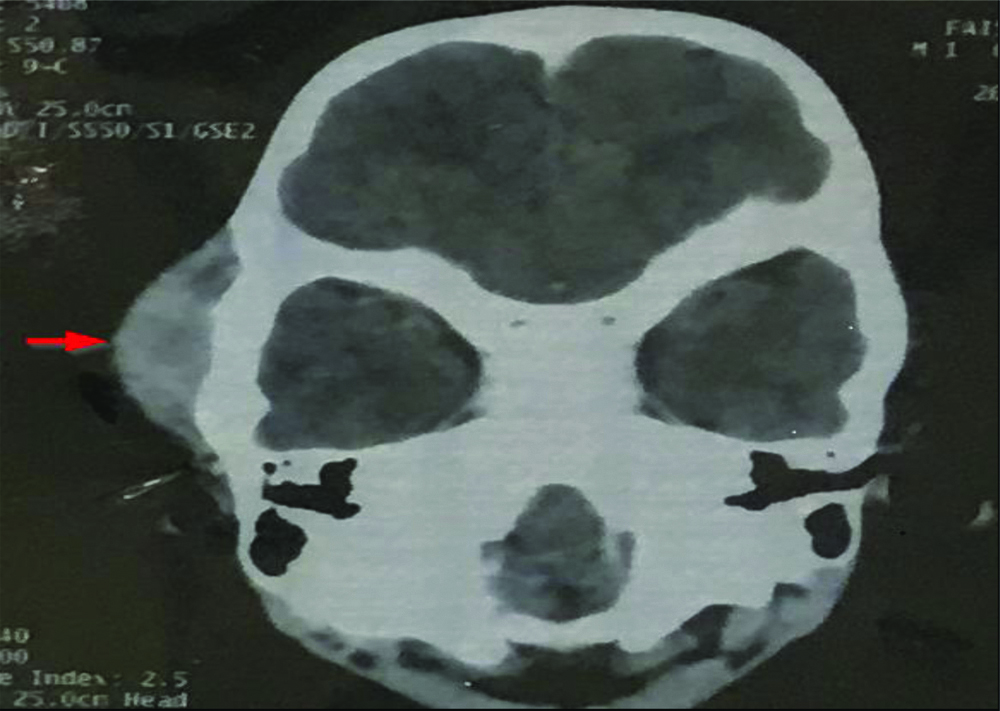
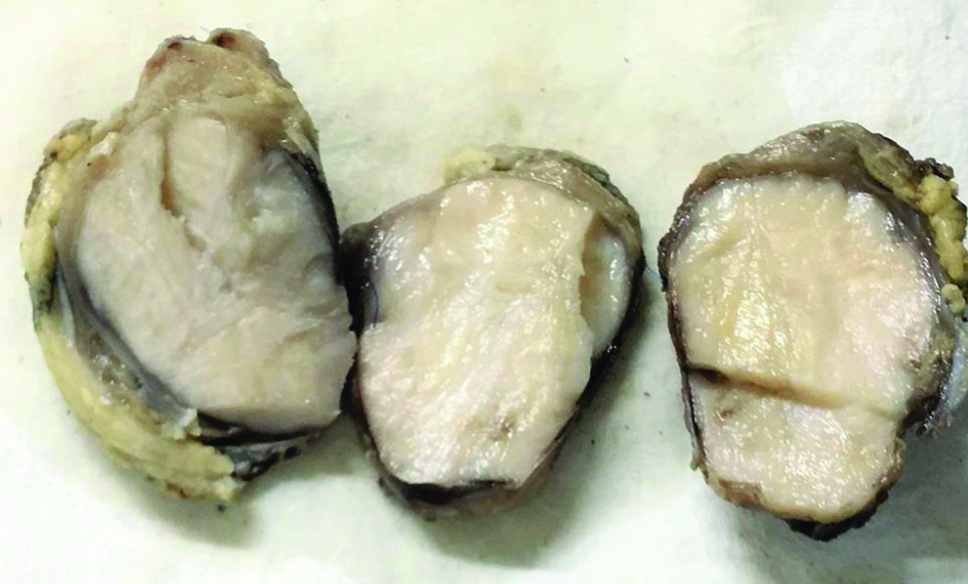
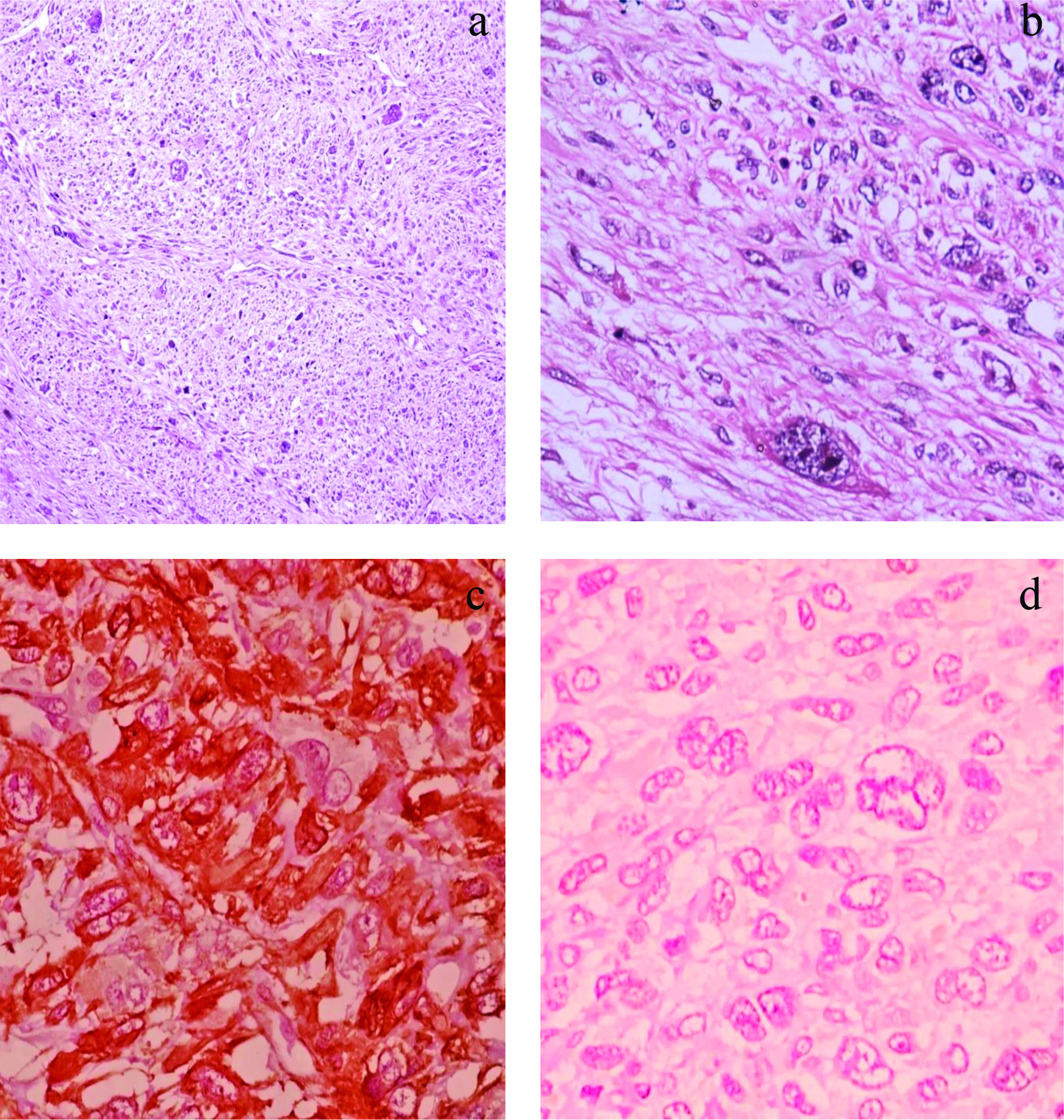
A one year and five-month-old male child presented with painless swelling in the right temporal region which gradually increased in size since one year with no other significant history. General physical examination was normal with no associated comorbidities. Serology for all viral markers including Human Immunodeficiency (HIV) was negative. The swelling was irregular, globular, firm on palpation with no fixity to skin. Contrast Enhanced Computed Tomography (CECT) face revealed a heterogeneously enhancing mass measuring 41×37 mm involving right infratemporal fossa and right temporalis muscle [Table/Fig-1]. Fine Needle Aspiration Cytology (FNAC) done at some other centre was reported as malignant small round cell tumour. The child was sent for surgical excision and further management to our hospital. Authors received excised temporal muscle with irregular globular mass measuring 5×3.5×4.5 cm. Cut surface appears solid grey-white and whorled in appearance [Table/Fig-2]. We also received part of tumour attached to bone and a single preauricular lymph node. Histopathological examination of the excised mass showed a highly cellular tumour with a pseudocapsule, comprising of spindle cells arranged in intersecting fascicles and showing focal palisading. Individual cells exhibited marked pleomorphism and had pale eosinophilic cytoplasm, elongated blunt ended vesicular nuclei with coarse granular chromatin and prominent nucleoli [Table/Fig-3a]. Bizarre multinucleated giant cells were also noted [Table/Fig-3b]. Brisk mitotic activity (4-5/ hpf) and few atypical mitotic figures were seen. Interspersed areas of necrosis and haemorrhage were present. Tumour along with bone was seen in the bony tissue received separately while the lymph node was not involved by tumour. A myriad of malignant spindle cell tumours were considered as differentials. LMS and Spindle Cell Rhabdomyosarcoma (SC-RMS) were high on the list followed by Malignant Peripheral Nerve Sheath Tumour (MPNST). On IHC, tumour cells stained positive for SMA [Table/Fig-3c] and Desmin and were negative for Myogenin [Table/Fig-3d] and S100. Ki67 was positive in 30% cells. A final diagnosis of LMS, Grade 2 was made. Further radiological investigation did not reveal any other lesion in the body. Cytogenetic study could not be performed. The patient was initiated on therapy however, due to aggressive course of disease the child succumbed in due course of time.
Contrast Enhanced Computed Tomography (CECT) face revealed a hetrogeneously enhancing mass involving right infratemporal fossa and right temporalis muscle (arrow).
Gross specimen of temporal muscle with irregular globular mass having solid grey-white whorled cut surface.
a) Areas displaying Leiomyosarcoma (LMS) morphology: Intersecting fascicles of spindle cells with elongated blunt ended nuclei and moderate eosinophilic cytoplasm along with few scattered pleomorphic giant cells (H&E, 100X). b) Tumour cells showing marked pleomorphism and bizarre cells (H&E, 400X). c) Tumor cells were diffusely positive for Smooth Muscle Actin (SMA) [SMA, 400X]. d) Tumor cells are negative for Myogenin [Myogenin, 400X].
Discussion
Soft tissue sarcomas of head and neck are rare tumours which account for 5-15% of all sarcomas [1]. LMS is a malignant tumour of smooth muscle origin commonly seen in adults and typically found in uterus and gastrointestinal tract. They are rarely encountered in children, comprising only 2% of their soft tissue sarcomas [2]. Head and neck LMS are believed to arise from smooth muscle cells found mainly in blood vessels, erector pile musculature of skin, circumvallate papilla, primitive mesenchyme and myoepithelial cells of salivary glands. Other modes of origin include aberrant mesenchymal differentiation and metastasis [3].
Yan B et al., documented a series of 20 cases of primary oral LMS, most frequently involving mandible and maxilla, cheek, soft palate and floor of mouth [4]. In other series, cases of LMS at various head and neck subsites like buccal mucosa, parapharyngeal space, maxilla, larynx, mandible and tongue have been reported [5].
Previously, case reports of rhabdomyosarcoma in the infratemporal fossa in young children have been documented [6]. However, within head and neck, LMS in the infratemporal fossa with involvement of temporalis muscle is virtually unheard of, barring a single reported case of paediatric oral LMS involving left masticator and buccal spaces including infratemporal fossa [7]. Although early presentation of LMS has not been attributed to any heritable syndromes, interestingly, AIDS related literature reveals a rising incidence of tumours of smooth muscle origin in HIV patients [8]. The present case was negative for HIV and other viral markers.
Keeping in mind patient’s age, location and histomorphology of the lesion, the closest differential in our case was SC-RMS. Grossly, these lesions vary in size from 1.5-35 cm [9]. On cut surface, they are firm, white to tan in colour, with whorled appearance. Histopathology shows cellular proliferation of elongated spindle cells arranged in fascicles or whorls with cells having blunt or fusiform ends, centrally located nuclei, small to inconspicuous or prominent nucleoli and eosinophilic fibrillar cytoplasm. All these features are reminiscent of LMS [10,11]. Admixed with this population we can get few rhabdomyoblasts showing eccentric nucleus, bright eosinophilic cytoplasm and on occasion cross striations which are pointers towards a tumour of skeletal muscle origin. These tumours show variable staining for smooth muscle markers like SMA and Desmin and thus, are of no significant help. Myogenin is more sensitive and specific than Desmin for these tumours and is useful in making a clear distinction from LMS [11,12]. Additionally, Myogenin shows no reactivity in other spindle cell proliferations, such as nodular fascitis, undifferentiated pleomorphic sarcoma, Malignant Peripheral Nerve Sheath Tumour (MPNST), inflammatory myofibroblastic tumour and myofibrosarcoma which may aid in the differential diagnosis [12]. In the current case, the lesion was poorly circumscribed measuring 5×3.5×4.5 cm and had grey-white, solid-cut surface with whorled appearance. Although morphology was confusing, lack of rhabdomyoblasts and negativity for Myogenin convincingly excluded spindle cell rhabdomyosarcoma. Other head and neck sarcoma differentials include fibrosarcoma, MPNST, pleomorphic sarcoma and angiosarcoma [1].
Infantile fibrosarcoma shows atypical spindle cells arranged in fascicles and classical herringbone pattern. However, they are frequently negative for SMA, Desmin and Myogenin which aids in distinction from LMS and SC-RMS. MPNST arises from peripheral nerve. In the head and neck region typically in younger patients, it is seen to be associated with type 1 Neurofibromatosis (NF1). These tumours show marbling pattern due to alternating hypocellular and hypercellular areas with perivascular accentuation and fairly uniform spindle cells [13]. All these features were absent in our case and S100 was negative thus, MPNST was excluded. In adults there are few other differentials common in the head and neck region such as spindle cell (sarcomatoid) squamous cell carcinoma, desmoplastic melanoma, pleomorphic sarcoma and angiosarcoma. These have characteristic morphological features and are usually negative for muscle markers.
Recent research reveals complex cytogenetic and molecular changes in LMS, most consistent changes being losses at regions 10q and 13q that harbour important tumour suppressor genes RB1 and PTEN and gains at 17p [14]. Considering that these tumours run a high risk of recurrence due to higher grade and precarious anatomical location, timely resection and therapy may alter the prognosis significantly.
Conclusion(s)
Leiomyosarcoma (LMS) are uncommon in the head and neck and have obscure childhood presentation; however, the possibility of their occurrence must not be overlooked owing to this rarity. This case highlights the pivotal role played by immunohistochemistry adjunct to histopathology in arriving at a correct diagnosis and thereby, facilitating treatment. It also brings to fore the importance of immunohistochemistry including Myogenin in excluding other entities within the spectrum of malignant spindle cell tumours of childhood.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 01, 2020
Manual Googling: Nov 12, 2020
iThenticate Software: Nov 24, 2020 (15%)
[1]. Bernadoy CG, Garrel R, Head and neck soft tissue sarcoma in adultsEur Ann Otorhinolaryngol 2016 133:37-42.10.1016/j.anorl.2015.09.00326403655 [Google Scholar] [CrossRef] [PubMed]
[2]. Lack EE, Leiomyosarcomas in childhood: A clinical and pathologic study of 10 casesPediatr Pathol 1986 6:181-97.10.3109/155138186090377113822934 [Google Scholar] [CrossRef] [PubMed]
[3]. Chen JM, Novick WH, Logan CA, Leiomyosarcoma of the larynxJ Otolaryngol 1991 20:345-48. [Google Scholar]
[4]. Yan B, Li Y, Pan J, Xia H, Li LJ, Primary oral leiomyosarcoma: A retrospective clinical analysis of 20 casesOral Dis 2010 16:198-203.10.1111/j.1601-0825.2009.01635.x20374505 [Google Scholar] [CrossRef] [PubMed]
[5]. Yadav J, Bakshi J, Chouhan M, Modi R, Head and neck leiomyosarcomaIndian J Otolaryngol Head Neck Surg 2013 65:01-05.10.1007/s12070-011-0305-824427607 [Google Scholar] [CrossRef] [PubMed]
[6]. Toufga Z, Fikri M, Jiddane M, Rhabdomyosarcoma of the infratemporal fossa: A case reportAustin J Radiol 2018 5:108010.26420/austinjradiol.2018.1080 [Google Scholar] [CrossRef]
[7]. Divyambika CV, Sathasivasubramanian S, Krithika CL, Malathi N, Prathiba D, Pediatric oral leiomyosarcoma: Rare case reportJ Can Res Ther 2012 8:282-85.10.4103/0973-1482.9899022842376 [Google Scholar] [CrossRef] [PubMed]
[8]. Purgina B, Rao UN, Miettinen M, Pantanowitz L, AIDS- Related EBV- Associated Smooth Muscle Tumours: A Review of 64 Published CasesPatholog Res Int 2011 2011:56154810.4061/2011/56154821437186 [Google Scholar] [CrossRef] [PubMed]
[9]. Nascimento AF, Fletcher CD, Spindle cell rhabdomyosarcoma in adultsAm J Surg Pathol 2005 29:1106-13.10.1097/01.pas.0000158396.57566.5d [Google Scholar] [CrossRef]
[10]. Goldblum JR, Folpe AL, Weiss SW, Enzinger and Weiss’s Soft Tissue Tumours 2019 7th edPhiladelphia, PAElsevier Saunders [Google Scholar]
[11]. Carroll SJ, Nodit L, Spindle cell rhabdomyosarcoma a brief diagnostic review and differential diagnosisArch Pathol Lab Med 2013 137:1155-58.10.5858/arpa.2012-0465-RS23899074 [Google Scholar] [CrossRef] [PubMed]
[12]. Cessna MH, Zhou H, Perkins SL, Tripp SR, Layfield L, Daines C, Are myogenin and myoD1 expression specific for rhabdomyosarcoma: A study of 150 cases, with emphasis on spindle cell mimicsAm J Surg Pathol 2001 25:1150-57.10.1097/00000478-200109000-0000511688574 [Google Scholar] [CrossRef] [PubMed]
[13]. Farid M, Demicco EG, Garcia R, Ahn L, Merola PR, Cioffi A, Malignant peripheral nerve sheath tumoursThe Oncologist 2014 19:193-201.10.1634/theoncologist.2013-032824470531 [Google Scholar] [CrossRef] [PubMed]
[14]. Serrano C, George S, LeiomyosarcomaHematol Oncol Clin North Am 2013 27:957-74.10.1016/j.hoc.2013.07.00224093170 [Google Scholar] [CrossRef] [PubMed]