Introduction
The ever-growing number of COVID-19 patients stresses upon the need to identify effective yet readily available predictors of disease severity to ensure better clinical outcomes. D-dimer is a fibrin specific degradation product derived by enzymatic action of plasmin on factor XIIIa cross-linked fibrin. It serves as an ideal marker for activation of coagulation and fibrinolytic pathways. Identification of coagulopathy as an important complication in COVID-19 patients has brought to focus D-dimer as a possible predictor of clinical severity in patients.
Aim
In this study, we analysed the role of D-dimer levels in assessing the clinical severity of the COVID-19 patients.
Materials and Methods
We enrolled 217 in-patients of Tirunelveli Medical College in this single centre observational study and classified them into asymptomatic, mild, moderate and severe according to “Clinical Management Protocol: COVID 19”, by the Ministry of Health and Family Welfare and Director General of Health Services. D-dimer was estimated in the separated plasma, using latex based assay using semi-automated coagulation analyser. Data were presented as percentages for categorical variables and median±Inter Quartile Range (IQR) for continuous variables. Chi-square test was used to compare the D-dimer values between symptomatic and asymptomatic groups. A value of p<0.05 was considered statistically significant.
Results
Among the 217 cases, 88.9% were asymptomatic cases, 8.8% presented with mild clinical severity and 2.3% had moderate clinical presentation. In our study population, the Mean±SD and Median±IQR of D-dimer values (in ng/mL) were 223.4±230.6 and 157.0±187.7, respectively. The mean D-dimer value was found to increase as the category of our study group ascended from asymptomatic patients to mild and moderate clinical cases. It was noted that 91.1% of the cases who had D-dimer values <500 ng/mL were asymptomatic. Also, the odds of patients with high levels of D-dimer being clinically symptomatic was 5.5 times more than the odds of patients with D-dimer levels <500 ng/mL.
Conclusion
Elevation of D-dimer levels associated with the severity of clinical course of patients infected with SARS CoV-2 when compared to patients with mild or asymptomatic clinical presentations.
Endothelial dysfunction, Factor XIIIa, Fibrinogen, Severity
Introduction
As of 25th September 2020, the ongoing pandemic of Coronavirus Disease 2019 (COVID-19) has affected 32,110,656 people worldwide including 980,031 deaths. In India alone, around 5,818,570 have contracted the disease till that date with mortality reaching 92,290 [1]. The Indian Ministry of Health and Family Welfare and Director General of Health Services published “Clinical Management Protocol: COVID 19” on 27th June 2020, which classifies the disease into mild, moderate and severe cases based on clinical manifestations [2]. Earlier reports from China suggest that approximately 26.1-32.0% of confirmed cases could progress to severe or critical cases [3,4]. The mortality rate is higher among patients with severe and critical illness than those with mild to moderate disease. Researchers have identified coagulopathy as an important complication in COVID-19 patients [5,6]. With this knowledge, researchers are focusing on D-dimer levels in COVID-19 patients with varying degrees of clinical presentation to assess its role as an early predictor of clinical prognosis [6-8].
During haemostasis, coagulation cascade gets activated, resulting in formation of thrombin, a key serine protein involved in clotting [9]. Next thrombin acts upon a 340 kDa soluble glycoprotein called fibrinogen. Fibrinogen molecule is a dimer composed of three pairs of three polypeptide chains which are held together by disulfide bonds. These chains are intertwined in such a way that the fibrinogen molecules consist of a central E domain linked by coiled-coil regions to two peripheral D domains [10]. The enzymatic activity of thrombin leads to the cleavage of two small fragments, fibrinopeptides A and B, from fibrinogen. This results in formation of fibrin monomer molecules. Also, there is conversion of negative charge present on the E-domain of fibrinogen into positive charge, causing these monomers to spontaneously polymerise into fibrin networks stabilised by hydrogen bonds. Meanwhile thrombin activates Factor XIII, which introduces covalent cross-links between the outer D domains of nearby monomers and the central E-domain of a third one causing further stabilisation of these polymers resulting in insoluble fibrin clots [11,12].
Under physiological conditions, the fibrin thrombi formed during coagulation undergo degradation as soon as they are formed by the fibrinolytic system. This is essential in order to maintain homeostatic balance [13]. The disintegration of fibrin clot begins with the formation of an important fibrinolytic component called plasmin. This is achieved by conversion of fibrin bound plasminogen to plasmin by tissue plasminogen activator, a serine protease released in response to tissue injury [14]. Plasmin mediated proteolysis of fibrinogen and fibrin produces multiple degradation products which are peptide fragments with a wide array of molecular weights [15]. Those fragments derived from fibrin polymers that underwent factor XIIIa mediated crosslinking will have intact covalent bonds bridging adjacent D domains and are called D-dimers [11,16]. Since D-dimer can only be produced when there is formation and degradation of cross-linked fibrin, it serves as a reactive marker of haemostatic balance [17]. D-dimer has a plasma half-life of about eight hours before clearance by kidneys and reticuloendothelial system [18]. Normal reference range of serum D-dimer is 220-740 ng/mL [19]. At present, D-dimer assays are extensively used in management of deep vein thrombosis, pulmonary embolism, Disseminated Intravascular Coagulation (DIC) and other thromboembolic conditions [11].
Even before the onset of this pandemic, D-dimer was speculated as a marker of organ dysfunction, need for intensive care unit admission and mortality in patients with suspected infection and sepsis [20,21]. Also, increased levels of D-dimer was noted in influenza like illness due to activation of coagulation by certain respiratory viruses [22]. Zhou F et al., and Tang N et al., studies showed D-dimer level >1000 ng/mL results in higher mortality in COVID-19 patients [6,7]. International Federation of Clinical Chemistry (IFCC) recommendations published in April, 2020 advocates testing of D-dimer levels in COVID-19 patients [23]. In this study, we analysed the role of D-dimer in assessing the clinical severity of the COVID-19 patients.
Materials and Methods
This is a single centre, cross-sectional study conducted at Government Tirunelveli Medical College, a Tertiary Care Centre in Southern India from March to July 2020. All those who had a contact history with COVID positive cases or clinical signs or symptoms were traced by the Public Health Care Workers and nasopharyngeal swab test was done in the ICMR approved Microbiology Laboratory of Tirunelveli Medical College. Those who tested positive for Real Time Polymerase Chain Reaction (RT-PCR) were admitted in the hospital and included in this study irrespective of their age. All those who were negative for RT-PCR were excluded. Thus, we enrolled 217 patients (N). This study was approved by the Ethics Committee of the Institute (REF NO: 1792/PATH/2020) and data was collected retrospectively. Based on the clinical data, they were classified. According to the “Clinical Management Protocol: COVID 19” by the Ministry of Health and Family Welfare and Director General of Health Services [2]. The clinical classification was as follows: 1) Asymptomatic: Contacts or patients with travel history tested swab positive by PCR, without any clinical signs like fever, sore throat, dry cough, dyspnoea; 2) Mild: Patients with uncomplicated upper respiratory tract infection may have mild symptoms such as fever, cough, sore throat, nasal congestion, malaise, headache without evidence of breathlessness or hypoxia (normal saturation); 3) Moderate: Pneumonia with no signs of severe disease. For adolescents or adults with presence of clinical features of dyspnoea and or hypoxia, fever, cough, including SpO2 <94% (range 90-94%) on room air, respiratory rate is more or equal to 24 breaths per minute. For children with presence of clinical features of dyspnoea and or hypoxia, fever, cough, including SpO2 <94% (range 90-94%) on room air, respiratory rate is more or equal to 40 breaths per minute. Fast breathing is defined as (in breaths/min): <2 months: ≥60; 2-11 months: ≥50; 1-5 years: ≥40; 4) Severe: Severe Pneumonia with clinical signs of pneumonia plus one of the following; respiratory rate >30 breaths/min, severe respiratory distress, SpO2 <90% on room air for adolescent or adult and child with cough or difficulty in breathing, plus at least one of the following: central cyanosis or SpO2 <90%; severe respiratory distress (e.g., grunting, chest indrawing); signs of pneumonia with any of the following danger signs: inability to breastfeed or drink, lethargy or unconsciousness, or convulsions; with any other following signs of pneumonia: chest indrawing, fast breathing (in breaths/min): <2 months ≥60; 2-11 months ≥50; 1-5 years ≥40. acute respiratory distress syndrome, sepsis, septic shock and death [2].
Data Collection and D-dimer Measurement
The patients travel history, exposure history, contact history, demographic data, signs and symptoms, laboratory and radiological data were collected. Peripheral venous blood samples were collected as per standard protocol in sodium citrate containers. D-dimer was estimated in the separated plasma, using latex based assay using semi-automated coagulation analyser (ECL-50, ERBA). Based on the Guan W et al., study (February 2020), the cut-off value for D-dimer level was fixed as 500 ng/mL and the study population are divided into two groups to compare their clinical severity [24]. The throat samples were tested for SARS COV2, with Labgun RT-PCR kit (Labgenomics, Republic of Korea) in the hospital laboratory. All collected samples were handled following strict biosafety measures and biomedical waste management guidelines.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) version 16.0 (SPSS Inc. Chicago, IL) was used. Percentages were used for categorical variables and Median±IQR for continuous variables. Chi-square test was used to compare the D-dimer values between symptomatic and asymptomatic groups. A value of p<0.05 was considered statistically significant.
Results
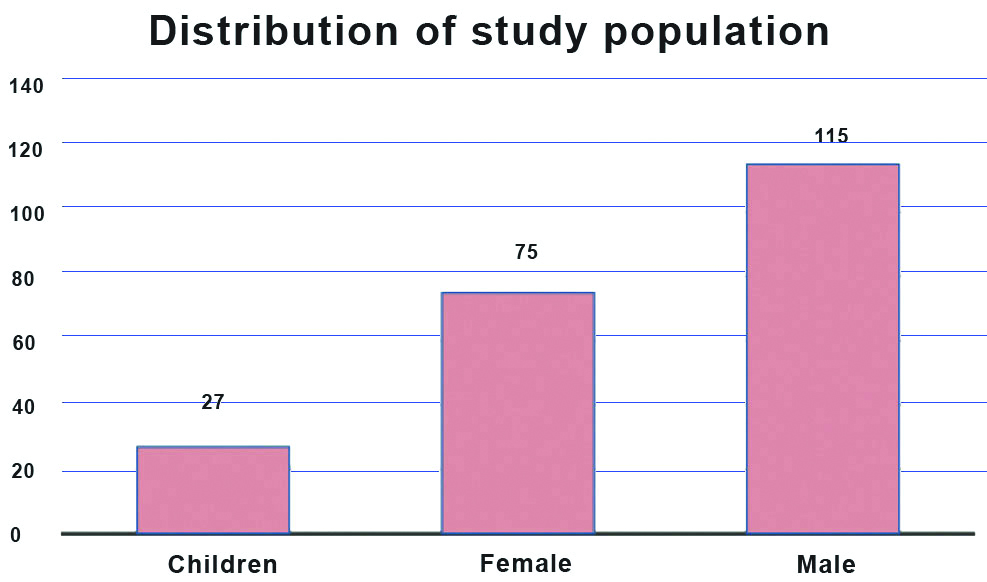
A total of 217 COVID-19 cases were included in the study population. A 87.5% (n=190) of the study population were adults and among them 52.9% (115) were male and 34.5% (75) were female [Table/Fig-1]. About 27 were children and in 31-40 and 41-50 age group there were 44 patients [Table/Fig-2]. Among the 217 cases 88.9% were asymptomatic cases, 8.8% were presented with mild clinical severity and 2.3% were with moderate clinical presentation [Table/Fig-3]. There was a statistically significant difference in age, Neutrophil count (%), RBC count and Neutrophil Lymphocyte (NL) Ratio between the symptomatic and asymptomatic cases [Table/Fig-4].
Distribution of study population.
| Frequency |
|---|
| Male | Female | Total |
|---|
| >70 years | 2 | 1 | 3 |
| 61-70 years | 14 | 13 | 27 |
| 51-60 years | 21 | 15 | 36 |
| 41-50 years | 26 | 18 | 44 |
| 31-40 years | 26 | 18 | 44 |
| 21-30 years | 17 | 6 | 23 |
| 13-20 years | 9 | 4 | 13 |
| <13 years | 27 |
Distribution of study subjects based on clinical presentation.
| Clinical grading | Frequency | Percent |
|---|
| Asymptomatic cases | 193 | 88.9 |
| Mild clinical presentation | 19 | 8.8 |
| Moderate clinical presentation | 5 | 2.3 |
Biochemical and haematological values in asymptomatic and symptomatic COVID-19 patients.
| n | Total cases (n=217) | Asymptomatic cases (n=193) | Symptomatic cases (n=24) | Mann-Whitney U test p-value |
|---|
| Median | IQR* | Median | IQR | Median | IQR |
|---|
| Age (years) | 217 | 36 | 27 | 36 | 29 | 42 | 22 | 0.04 |
| Blood glucose (mg/dL) | 206 | 88 | 38.5 | 88 | 39 | 91.5 | 72.8 | 0.95 |
| Serum total protein (g/dL) | 198 | 7 | 0.5 | 7 | 0.5 | 6.9 | 0.8 | 0.23 |
| Sodium (mEq/L) | 193 | 140 | 4 | 139 | 4 | 139.5 | 3.8 | 0.80 |
| Potassium (mEq/L) | 193 | 4 | 0.4 | 4 | 0.4 | 4 | 0.4 | 0.45 |
| CK† (U/L) | 195 | 74 | 90.3 | 77.6 | 78 | 49 | 64.1 | 0.09 |
| LDH‡ (U/L) | 195 | 403 | 146.8 | 399 | 153 | 456 | 131.3 | 0.76 |
| Ferritin (ng/mL) | 165 | 112 | 144.3 | 107 | 135 | 140.5 | 301.3 | 0.22 |
| Total WBC§ count (cells/mm3) | 206 | 7550 | 3400 | 7500 | 3100 | 6900 | 4700 | 0.41 |
| Neutrophil (%) | 206 | 56 | 15 | 54 | 15 | 60.5 | 21 | 0.02 |
| Lymphocytes (%) | 206 | 36 | 15 | 38 | 14 | 38.5 | 20 | 0.06 |
| Eosinophil (%) | 205 | 7 | 5 | 8 | 5 | 6 | 5 | 0.07 |
| NL Ratio|| | 206 | 1.5 | 1.1 | 1.48 | 1.03 | 1.8 | 2.43 | 0.03 |
| RBC** (million/mm3) | 206 | 4.8 | 0.59 | 4.78 | 0.59 | 4.54 | 0.66 | 0.01 |
| HB†† (g/dL) | 206 | 13.3 | 2.6 | 13.3 | 2.5 | 13.3 | 3.9 | 0.70 |
| PCV‡‡ (%) | 206 | 38.2 | 6.6 | 38.2 | 6.6 | 37.8 | 8.3 | 0.44 |
| Platelet (Lacs) | 203 | 2.8 | 1.09 | 2.82 | 1.09 | 2.73 | 0.99 | 0.35 |
*IQR: Inter quartile range; †CK: Creatine kinase; ‡LDH: Lactate dehydrogenase; §WBC: White blood cell; ||NLRatio: Neutrophil lymphocyte ratio; **RBC: Red blood cell; ††HB: Haemoglobin; ‡‡PCV: Packed cell volume
In our study population, the Mean±SD and Median±IQR of D-dimer values (in ng/mL) were 223.4±230.6 and 157.0±187.7, respectively. The mean D-dimer value was 212.7 ng/mL in asymptomatic cases which increases to 243.7 ng/mL in mild cases and 525.8 ng/mL in patients with moderate clinical presentation. The mean D-dimer value was found to increase as the category of our study group ascended from asymptomatic patients to mild, moderate clinical cases [Table/Fig-5]. Around 91.1% of the cases who had normal D-dimer values (<500 ng/mL) were asymptomatic cases [Table/Fig-6]. The Chi-square test showed that it was statistically significant and the odds of patients with high level of D-dimer were supposed to be clinically symptomatic cases which were 5.5 times more than that of the odds of patients with normal levels of D-dimer [Table/Fig-7].
D-dimer in asymptomatic cases, mild and moderate cases.
| Clinical severity | N | Mean (ng/mL) | SD | Median (ng/mL) | IQR |
|---|
| Asymptomatic cases | 193 | 212.7 | 216.3 | 151.0 | 183.9 |
| Mild clinical presentation | 19 | 243.7 | 212.6 | 164.0 | 287.4 |
| Moderate clinical presentation | 5 | 557.8 | 525.8 | 556.0 | 858.4 |
Cross tabulation between clinical severity and D-dimer grading.
| D-dimer category | Patients |
|---|
| Symptomatic cases | Asymptomatic cases |
|---|
| Normal (<500 ng/mL) | Count | 18 | 182 |
| % within D-dimer category | 9.0% | 91.0% |
| High (>500 ng/mL) | Count | 6 | 11 |
| % within D-dimer category | 35.3% | 64.7% |
| Value | p-value |
|---|
| Pearson chi-square | 11.013 | 0.001 |
| Odds ratio for D-dimer category (High/Normal) | 5.5 |
Discussion
A recent article by Iba T et al., presents a better overview about the possible pathogenesis. Persistent inflammation results in the formation of Interleukin-1β and Interleukin-6 which are known to cause thrombocytosis and hyperfibrinogenemia. In the early stage of disease, inflammation and coagulation are limited to the lungs. But as disease progresses, these features become systemic and later on present as Disseminated Intravascular Coagulation (DIC). Alveolar macrophages release urokinase type Plasminogen Activator (u-PA) which promotes local fibrinolysis and D-dimer elevation. Moreover, direct infection of endothelium by the SARS-CoV2 virus via its receptor, Angiotensin-Converting Enzyme 2 (ACE-2), results in a surge in plasminogen activator release. As disease severity progresses, increased fibrinogen levels and activated platelets aggravates procoagulant state. Pulmonary microthrombi formation is promoted by Plasminogen Activator Inhibitor 1 (PAI-1) which leads to suppression of fibrinolysis. In normal vascular endothelium ACE-2 mediates anticoagulant activities. But once SARS-CoV2 virus binds to it, ACE-2 causes cell damage followed by increased expression of tissue factor and downregulation of the protein C system. Ultimately coagulation and thrombotic events may occur even in the absence of secondary complications like tissue hypoxia and superadded infections [25].
Studies conducted in Netherland and France show increased incidence of thrombotic complications in patients with severe disease [26,27]. A retrospective study on 183 patients by Tang N et al., established that 71.4% of non-survivors and around 0.6% of the survivors showed features of overt DIC. The median time from admission to development of DIC was four days [7]. Autopsy findings published by Wichmann D et al., brings to notice a high incidence of thromboembolism in COVID-19 patients and the need to consider pulmonary embolism in cases of haemodynamic instability [28]. A state of dynamic hypercoagulation as evidenced by the presence of microthrombi in various organs accompanied by reduced platelet levels is seen in COVID patients, especially those with comorbidities. Plasmin mediated hyperactive fibrinolysis may also lead to haemorrhage and marked elevation of circulating fibrin degradation products in such patients [29].
In our study, we noted that the mean D-dimer value increases as the category of our study group moves up from asymptomatic patients to mild, moderate and severe clinical cases. The mean D-dimer value was 212.7 ng/mL in asymptomatic cases which increases to 243.7 ng/mL in mild cases and 525.8 ng/mL in patients with moderate clinical presentation. These findings are in concordance with a multicentre retrospective study conducted in China by Guan W et al., [24]. According to it, 260 of the 560 enrolled patients amounting to 46.4% had elevated D-dimer levels (0.5 mg/L) and this rise in value was more pronounced among severe patients when compared to non-severe ones. They defined the degree of severity of COVID-19 as severe cases if patients admitted with any one of the following major criteria: septic shock with need for vasopressors; respiratory failure requiring mechanical ventilation or any three or more of the following minor criteria: respiratory rate >30 breaths/min; PaO2/FIO2 ratio <250; multilobar infiltrates; confusion/disorientation; uraemia (blood urea nitrogen level >20 mg/dL); leukopenia (white blood cell count, 4,000 cells/mL); thrombocytopenia (platelet count, 100,000/mL); hypothermia (core temperature, 36°C); Hypotension requiring aggressive fluid resuscitation [24]. Study by Tang N et al., in march 2020, showed that patients with severe disease had a 3.5 fold increase in D-dimer levels when compared to those without it along with higher mortality rates [7]. Similar findings were also reported by Yao Y et al., who observed a high level of serum D-dimer level in non-survivors when compared to the survivors [30].
A recent guidance report by International Society of Thrombosis and Haemostasis (ISTH) for recognition and management of coagulopathy in COVID-19 states markedly raised D-dimers levels, that is, three-four folds increase as a criteria for admission even though patient has no other severity symptoms [31]. We further established two groups in our study, one whose D-dimer value was above 500 ng/mL and another with values below 500 ng/mL. In our study, population, it was noted that 91.1% of the cases who had normal D-dimer values were asymptomatic. The chi-square test showed that it was statistically significant and the odds of a patient with high D-dimer levels turning out to be clinically symptomatic were 5.5 times more than that of the odds of a patient with normal D-dimer levels.
High D-dimer levels have been reported as one of the risk factors to predict occurrence of acute respiratory distress syndrome and also its progression to death in COVID-19 patients [32]. Wang D et al., noted that patients in need of admission to intensive care units had significantly higher D-dimer levels than those not in need of it [33]. Also, D-dimer levels show a sequential rise with time in non-survivors as against values in those who survived [6,33]. Zhang L et al., established a cut-off value of 2.0 μg/mL as an independent predictor for in-hospital death in patients [5].
Initiation of DIC in patients with sepsis is well-documented [34]. The pathophysiology behind this is considered to be endothelial dysfunction along with altered immune regulation [35]. Giannis et al., attributed this to endothelial dysfunction characterised by high levels of von Willebrand (vW) factor, a procoagulatory state due to activation of tissue factor pathway and toll-like receptor activation causing systemic inflammation in cases of coronavirus infections [36]. Increase in D-dimer levels in COVID-19 may be explained by excess production of thrombin and early shutdown of fibrinolytic pathway secondary to any infectious stimuli as noted in past studies [37]. Another possible explanation for this is hypoxia in severe cases of COVID-19 leading to thrombus formation via increased blood viscosity and hypoxia inducible transcription factor dependent signalling pathway [38]. Some suggest that high levels of circulating proinflammatory cytokines implicated in the cytokine storm noticed in severe cases of COVID-19 might lead to direct activation of coagulation cascade [29].
Limitation(s)
COVID-19 is a pandemic disease for which prevalence rate is not known. Due to the emerging need for the new classifying factors and new prognostic factors, data over the period of five months have been documented in this study. The current study was conducted at a single centre with limited sample size. Hence, further study may be needed to extrapolate the findings to wider patient population.
Conclusion(s)
Elevated D-dimer levels associated with the severity of clinical course of patients infected with SARS CoV-2 when compared to patients with mild or asymptomatic clinical presentations. Hence, this reinforces the fact that D-dimer levels at the time of diagnosis helps to predict the clinical course of the disease and is a definite guide for early management of complications. The prevalence of COVID-19 is not clearly documented and our present knowledge confines to only patients with moderate and severe clinical presentation who reported to hospital for treatment and diagnosed as COVID positive. This enhanced level of D-dimer is a definite indicator of the pathogenetic mechanisms underlying severe clinical presentations and a subject matter of deeper analysis. The role of vW Factor in the increase of D-dimer levels in severely affected COVID-19 patients needs further study.
*IQR: Inter quartile range; †CK: Creatine kinase; ‡LDH: Lactate dehydrogenase; §WBC: White blood cell; ||NLRatio: Neutrophil lymphocyte ratio; **RBC: Red blood cell; ††HB: Haemoglobin; ‡‡PCV: Packed cell volume
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 17, 2020
Manual Googling: Oct 17, 2020
iThenticate Software: Oct 29, 2020 (18%)
[1]. WHO Coronavirus Disease (COVID-19) Dashboard [Internet]. [cited 2020 Jul 4]. Available from: https://covid19.who.int [Google Scholar]
[2]. Clinical Management Protocol for COVID19 dated 27062020.pdf [Internet]. [cited 2020 Jul 18]. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19dated27062020.pdf [Google Scholar]
[3]. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive studyThe Lancet 2020 395(10223):507-13.10.1016/S0140-6736(20)30211-7 [Google Scholar] [CrossRef]
[4]. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, ChinaLancet 2020 395:497-506.10.1016/S0140-6736(20)30183-5 [Google Scholar] [CrossRef]
[5]. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19J Thromb Haemost JTH 2020 18(6):1324-29.10.1111/jth.1485932306492 [Google Scholar] [CrossRef] [PubMed]
[6]. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort studyThe Lancet 2020 395(10229):1054-62.10.1016/S0140-6736(20)30566-3 [Google Scholar] [CrossRef]
[7]. Tang N, Li D, Wang X, Sun Z, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumoniaJ Thromb Haemost JTH 2020 18(4):844-47.10.1111/jth.1476832073213 [Google Scholar] [CrossRef] [PubMed]
[8]. Gao Y, Li T, Han M, Li X, Wu D, Xu Y, Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19J Med Virol 2020 92(7):791-96.10.1002/jmv.2577032181911 [Google Scholar] [CrossRef] [PubMed]
[9]. Ramachandran R, Hansen KK, Hollenberg MD, Proteinase-Activated Receptors. In: Lennarz WJ, Lane MD, editorsEncyclopedia of Biological Chemistry (Second Edition) [Internet] 2013 WalthamAcademic Press:60-06.Available from: http://www.sciencedirect.com/science/article/pii/B978012378630200398410.1016/B978-0-12-378630-2.00398-4 [Google Scholar] [CrossRef]
[10]. Hoeprich PDJ, Doolittle RF, Dimeric half-molecules of human fibrinogen are joined through disulfide bonds in an antiparallel orientationBiochemistry 1983 22(9):2049-55.10.1021/bi00278a0036860649 [Google Scholar] [CrossRef] [PubMed]
[11]. Riley RS, Gilbert AR, Dalton JB, Pai S, McPherson RA, Widely used types and clinical applications of D-Dimer assayLab Med 2016 47(2):90-102.10.1093/labmed/lmw00127016528 [Google Scholar] [CrossRef] [PubMed]
[12]. Wakai A, Gleeson A, Winter D, Role of fibrin D-dimer testing in emergency medicineEmerg Med J 2003 20(4):319-25.10.1136/emj.20.4.31912835339 [Google Scholar] [CrossRef] [PubMed]
[13]. Gaffney PJ, Edgell TA, Whitton CM, The haemostatic balance- astrup revisitedPathophysiol Haemost Thromb 1999 29(1):58-71.10.1159/00002246110494035 [Google Scholar] [CrossRef] [PubMed]
[14]. Longstaff C, Kolev K, Basic mechanisms and regulation of fibrinolysisJ Thromb Haemost 2015 13(S1):S98-105.10.1111/jth.1293526149056 [Google Scholar] [CrossRef] [PubMed]
[15]. Gaffney PJ, Fibrin degradation products. A review of structures found in vitro and in vivoAnn N Y Acad Sci 2001 936:594-610.10.1111/j.1749-6632.2001.tb03547.x [Google Scholar] [CrossRef]
[16]. Adam SS, Key NS, Greenberg CS, D-dimer antigen: Current concepts and future prospectsBlood 2009 113(13):2878-87.10.1182/blood-2008-06-16584519008457 [Google Scholar] [CrossRef] [PubMed]
[17]. Weitz JI, Fredenburgh JC, Eikelboom JW, A Test in Context: D-DimerJ Am Coll Cardiol 2017 70(19):2411-20.10.1016/j.jacc.2017.09.02429096812 [Google Scholar] [CrossRef] [PubMed]
[18]. Hager K, Platt D, Fibrin degeneration product concentrations (D-Dimers) in the course of ageingGerontology 1995 41(3):159-65.10.1159/0002136777601368 [Google Scholar] [CrossRef] [PubMed]
[19]. Kratz A, Pesce MA, Basner RC, Einstein AJ, Appendix: Laboratory Values of Clinical ImportanceIn: Harrison’s Principles of Internal Medicine 2015 19th editionNew YorkMcGraw-Hill Education:2754 [Google Scholar]
[20]. Garcia-Olivé I, Sintes H, Radua J, Abad Capa J, Rosell A, D-dimer in patients infected with COVID-19 and suspected pulmonary embolismRespir Med 2020 169:10602310.1016/j.rmed.2020.10602332454268 [Google Scholar] [CrossRef] [PubMed]
[21]. Goebel PJ, Williams JB, Gerhardt RT, A pilot study of the performance characteristics of the D-dimer in presumed sepsisWest J Emerg Med 2010 11(2):173-79. [Google Scholar]
[22]. Wissen MV, Keller TT, Gorp ECMV, Gerdes VEA, Meijers JCM, Doornum GJJV, Acute respiratory tract infection leads to procoagulant changes in human subjectsJ Thromb Haemost 2011 9(7):1432-34.10.1111/j.1538-7836.2011.04340.x21605331 [Google Scholar] [CrossRef] [PubMed]
[23]. IFCC Information Guide on COVID-19 Tuesday 14 April updates- IFCC [Internet]. [cited 2020 Jul 4]. Available from: https://www.ifcc.org/ifcc-news/2020-03-26-ifcc-information-guide-on-covid-19/ [Google Scholar]
[24]. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, Clinical Characteristics of Coronavirus Disease 2019 in ChinaN Engl J Med 2020 382(18):1708-20.10.1056/NEJMoa200203232109013 [Google Scholar] [CrossRef] [PubMed]
[25]. Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M, The unique characteristics of COVID-19 coagulopathyCrit Care 2020 24(1):36010.1186/s13054-020-03077-032552865 [Google Scholar] [CrossRef] [PubMed]
[26]. Julien P, Julien G, Morgan C, Erika P, Thibault D, Fanny L, Pulmonary Embolism in Patients With COVID-19Circulation 2020 142(2):184-86.10.1161/CIRCULATIONAHA.120.04743032330083 [Google Scholar] [CrossRef] [PubMed]
[27]. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Incidence of thrombotic complications in critically ill ICU patients with COVID-19Thromb Res 2020 191:145-47.10.1016/j.thromres.2020.04.01332291094 [Google Scholar] [CrossRef] [PubMed]
[28]. Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Autopsy findings and venous thromboembolism in patients with COVID-19Ann Intern Med [Internet] 2020 May 6 [cited 2020 Jul 18] Available from: https://doi.org/10.7326/M20-200310.7326/M20-200332374815 [Google Scholar] [CrossRef] [PubMed]
[29]. Ji HL, Zhao R, Matalon S, Matthay MA, Elevated plasmin (ogen) as a common risk factor for COVID-19 susceptibilityPhysiol Rev 2020 100(3):1065-75.10.1152/physrev.00013.202032216698 [Google Scholar] [CrossRef] [PubMed]
[30]. Yao Y, Cao J, Wang Q, Shi Q, Liu K, Luo Z, D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control studyJ Intensive Care 2020 8(1):4910.1186/s40560-020-00466-z32665858 [Google Scholar] [CrossRef] [PubMed]
[31]. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, ISTH interim guidance on recognition and management of coagulopathy in COVID-19J Thromb Haemost 2020 18(5):1023-26.10.1111/jth.1481032338827 [Google Scholar] [CrossRef] [PubMed]
[32]. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, ChinaJAMA Intern Med 2020 180(7):934-43.10.1001/jamainternmed.2020.099432167524 [Google Scholar] [CrossRef] [PubMed]
[33]. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, ChinaJAMA 2020 323(11):1061-69.10.1001/jama.2020.158532031570 [Google Scholar] [CrossRef] [PubMed]
[34]. Voves C, Wuillemin WA, Zeerleder S, International Society on Thrombosis and Haemostasis score for overt disseminated intravascular coagulation predicts organ dysfunction and fatality in sepsis patientsBlood Coagul Fibrinolysis 2006 17(6):445-51.10.1097/01.mbc.0000240916.63521.2e16905947 [Google Scholar] [CrossRef] [PubMed]
[35]. Lillicrap D, Disseminated intravascular coagulation in patients with 2019-nCoV pneumoniaJ Thromb Haemost JTH 2020 18(4):786-87.10.1111/jth.1478132212240 [Google Scholar] [CrossRef] [PubMed]
[36]. Giannis D, Ziogas IA, Gianni P, Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the pastJ Clin Virol 2020 127:10436210.1016/j.jcv.2020.10436232305883 [Google Scholar] [CrossRef] [PubMed]
[37]. Levi M, Poll T van der, Coagulation and sepsisThromb Res 2017 149:38-44.10.1016/j.thromres.2016.11.00727886531 [Google Scholar] [CrossRef] [PubMed]
[38]. Gupta N, Zhao YY, Evans CE, The stimulation of thrombosis by hypoxiaThromb Res 2019 181:77-83.10.1016/j.thromres.2019.07.01331376606 [Google Scholar] [CrossRef] [PubMed]