Breast cancer represents a diverse group of tumours that vary in clinical behaviour and response to therapy. It is the leading cause of morbidity and mortality among females in Indian metropolitan cities [1]. Invasive carcinoma of no special type is the largest group of malignant mammary tumour comprising of 75% of mammary carcinoma [2], whereas AC is a very rare breast cancer constituting only 0.3-1.0% of all breast cancers [3]. Diagnosis of ductal NST is done when features of other subtypes are not found. So, diagnosis of IDC-NST is done by diagnosis of exclusion. Microscopically, tumour cells are in diffuse sheets, nests, cords, singles and some are with ductal differentiation. Foci of squamous metaplasia, apocrine metaplasia or clear cell changes can be seen sometimes. However, apocrine cells in Ductal NST are <90% of tumour cells. For the diagnosis of AC, more than 90% of tumour cells should show cytologic and immunohistochemical features of apocrine cells [4]. Microscopically, AC and invasive breast carcinoma-NST (Ductal NST) show same architectural pattern, differing only in their cytological appearance. The tumour cells in AC have abundant acidophilic granular cytoplasm with eosinophilic or golden brown granules, vesicular nuclear chromatin with prominent nucleoli [5]. Apocrine snouts i.e., bulbous expansion of luminal tumour cells towards the lumen are also found. AC have altered hormone receptor pattern like low expression of Estrogen Receptor (ER) and Progesterone Receptor (PR) with over expression of Androgen Receptor (AR) and strong reactivity for GCDFP-15 [6,7]. This study was undertaken to know the incidence of AC in patients of VIMSAR, Burla and to analyse them with respect to different clinicopathological features and compare them with that of IDC-NST.
Materials and Methods
This was an observational study done retrospectively on 670 patients diagnosed as invasive breast carcinoma in Department of Pathology, Veer Surendra Sai Institute of Medical Science and Research, Burla between March 2010 to February 2019. Male patients with breast carcinoma and female patients taking neoadjuvant chemotherapy were excluded from study group. Clinicopathological features of AC were compared with invasive breast carcinoma NST. Patient age, clinical presentation, average size of tumour, lymph node status, modified Bloom-Richardson (BR) grading, hormone receptor status like ER, PR, HER2/neu, Ki-67, p53 were taken into consideration for both AC and IDC-NST. Immunoreactivity to CK-7 and GCDFP-15 were done for suspected AC in an outside laboratory.
Gross findings of received specimens were noted from histology registers of Pathology Department, VIMSAR, Burla. Formalin-fixed paraffin embedded tissue sections of 3-4 μm thickness from archived paraffin blocks were taken out for study. Haematoxylin-eosin stained slides were evaluated for cell architecture, cell morphology, nuclear features, necrosis and mitosis.
A 10% neutral buffered formalin fixed paraffin embedded tissue sections were steamed for antigen retrieval with 10 mM citrate buffer (pH 6.0) for 30 minutes. Following protein block, slides were incubated with antibody for ER, PR, HER2/neu, Ki-67, P53 by DAKO envision flex/Horseradish Peroxidase (HRP) antigen reagent. A 3, 3’-Diaminobenzidine Tetrahydrochloride (DAB) was used for the visualisation of antibody/enzyme complex. Slides were counterstained with haematoxylin and examined by light microscopy. ER and PR positivity was defined when >1% tumour cell nuclei stained positive for these hormone receptors. Ki-67 immunostain was used to assess proliferation rate. A 500 tumour cells were counted and Ki-67 was expressed as percentage of positive cells showing nuclear staining. Ki-67 index ≥20% was considered positive and <20% as negative. p53 was considered positive, when ≥10% cancer cell nuclei expressed it. Intense and complete membrane staining in >10% of tumour cells was labelled as HER2 overexpression. Immunoreactivity was scored 0=negative, 1=weak positive, 2=moderate and 3=strong positive in ≥30% tumour cells. HER2 immunostain 3/3 was considered HER-2 positive.
The CK-7 (ready to use; DAKO) and GCDFP-15 (ready to use; DAKO) results were availed from an outsourced laboratory in suspected cases of AC. Cytoplasmic reactivity of GCDFP-15 in >10% tumour cells was taken as positive. CK-7 was considered positive, if ≥1% tumour cells were stained with at least mild intensity in cytoplasm.
Statistical Analysis
Statistical data were analysed by Statistical Package For The Social Sciences (SPSS) version 23.0 for windows. The generated data was entered in a designed excel sheet for window. Data were presented in number followed by percentage. The categorical data was compared by Pearson’s χ2 test. Results having p-value <0.05 were accepted as significant.
Results
Total 670 invasive breast carcinoma cases were found from March 2010 to February 2019. There were 560 (83.58%) of IDC-NST cases and 5 (0.74%) of AC cases. Rest 105 (15.67%) cases included lobular carcinoma, metaplastic carcinoma, medullary carcinoma, papillary carcinoma, etc. The clinical features of AC and of IDC-NST cases are summarised in [Table/Fig-1].
Clinical features of Apocrine Carcinoma (AC) and Invasive Duct Carcinoma-No Special Type (IDC-NST).
| Clinical features | Apocrine Carcinoma (AC) (n=05) | IDC-NST (n=560) | Chi-square value |
|---|
| Mean age (Years) | 59.5 | 50.4 | |
| Tumour size (in cm) | ≤5 cm | 02 (40%) | 196 (35%) | χ2=0.054;p=0.815 |
| >5 cm | 03 (60.0%) | 364 (65%) |
| Lymphadenopathy | Present | 2 (40%) | 468 (83.5%) | χ2=3.972;p=0.046 |
| Absent | 03 (60.0%) | 92 (16.4%) |
| Discharge from skin | Present | 01 (20%) | 198 (35.35%) | χ2=0.5123;p=0.474 |
| Absent | 04 (80%) | 362 (64.64%) |
Mean age of AC patients at the time of diagnosis was 59.5 years and that of IDC-NST was 50.4 years. None of the AC, presented with nipple discharge whereas 198 cases (35.35%) of IDC-NST had complain of nipple discharge, both serous and bloody. Only one AC case had serous discharging sinuses in skin over breast (p=0.474). Breast lump of size ≤5 cm was found in 2 cases (40%) of AC and 196 cases (35%) of IDC-NST cases (p=0.815). Lymphadenopathy was found in 2 (40%) of the AC cases, but it was present in 468 (83.5%) of IDC-NST cases (p=0.046). Results of pathological examinations are given in [Table/Fig-2].
| Pathological features | Apocrine Carcinoma (AC) (n=05) | IDC-NST (n=560) | Chi-square value |
|---|
| Nodal metastasis | Present | 02 (40%) | 448 (80%) | χ2=4.8912;p=0.026 |
| Absent | 03 (60%) | 112 (16.4%) |
| Modified BR grade | I | 01 (20%) | 64 (11.42%) | χ2=1.0053;p=0.605 |
| II | 03 (60%) | 267 (47.67%) |
| III | 01 (20%) | 229 (40.89%) |
IDC-NST: Invasive duct carcinoma-no special type; BR: Bloom-Richardson
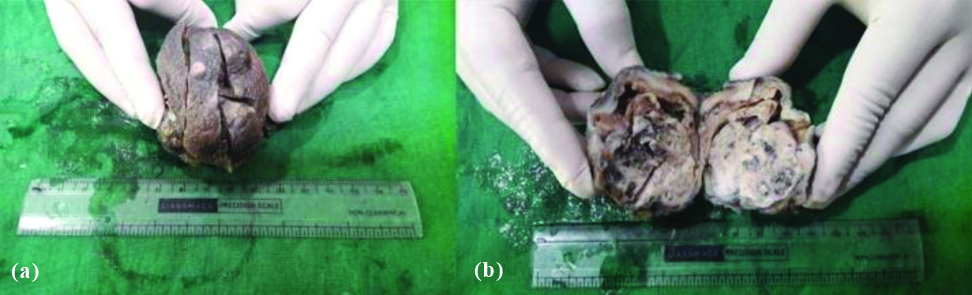
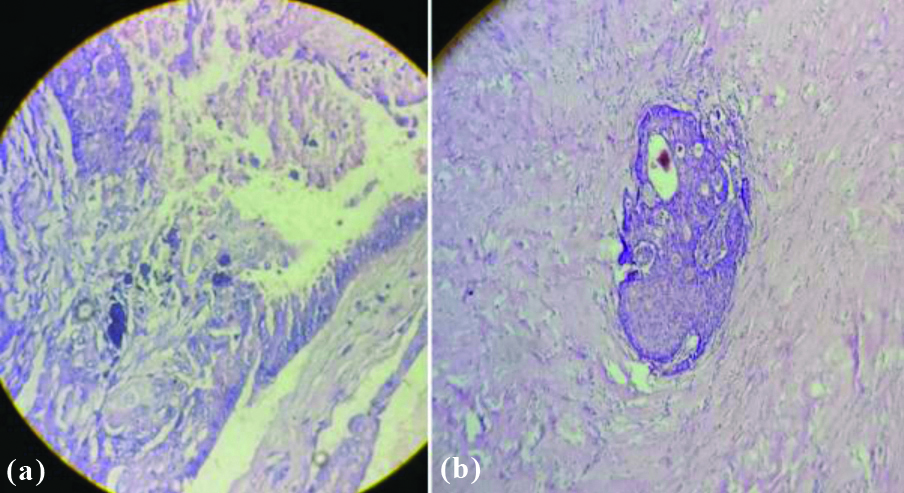
Grossly, no significant difference was observed between AC and IDC-NST, except the size of tumour which was more in IDC-NST. Cut-section of mass in both IDC-NST and AC showed both solid and cystic areas with friable growth at some focus along with areas of haemorrhage and necrosis [Table/Fig-3a,b]. Microscopic examination of H&E stained tissue sections showed 3 (60%) AC cases and 267 (47.67%) cases of IDC-NST to be of grade II (p=0.605). Pattern of tumour cells were similar in both IDC-NST and AC, but the morphology of tumour cells was different in AC. The cells in AC were round to polygonal cells with abundant granular eosinophilic cytoplasm and round to oval nucleus with prominent nucleoli. Stromal invasion and cytoplasmic snouts found projecting into the cyst lumen [Table/Fig-4a,b]. Lymph nodes were dissected in 468 (83.5%) cases of IDC-NST and 2 (40%) cases of AC. Both the cases of AC showed metastatic deposits in lymph nodes. However, metastatic deposits in lymph nodes were seen in 448 (80%) cases of IDC-NST (p=0.026). Result of IHC is given in [Table/Fig-5].
Gross photo of AC of breast showing: (a) globular mass with multiple sinuses on skin; (b) Cut-section shows pushing margin, solid and cystic areas.
Microsection showing (a) apocrine snout, type A cells at left upper corner and type B cells at left lower corner with central area of calcification in AC. (b) Stromal invasion seen in AC (H&E stain, 400 x).
Immunohistochemical (IHC) features.
| IHC findings | Apocrine carcinoma (AC) (n=05) | IDC-NST (n=560) | Chi-square value |
|---|
| p53 | Positive | 03 (60.0%) | 157 (28.03%) | χ2=2.494;p=0.114 |
| Negative | 02 (40%) | 403 (71.96%) |
| Ki-67 | Positive | 05 (100%) | 279 (49.8%) | χ2=3.186;p=0.0743 |
| Negative | 00 (0%) | 281 (50.1%) |
| Basal like molecular subtype | 03 (60.0%) | 213 (38.2%) | |
IDC-NST: Invasive duct carcinoma-no special type
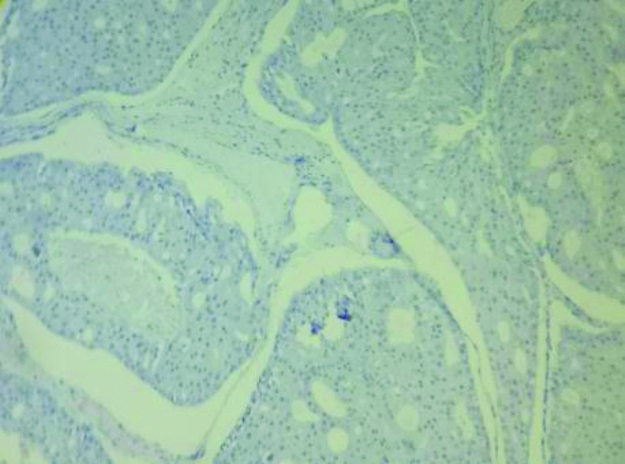
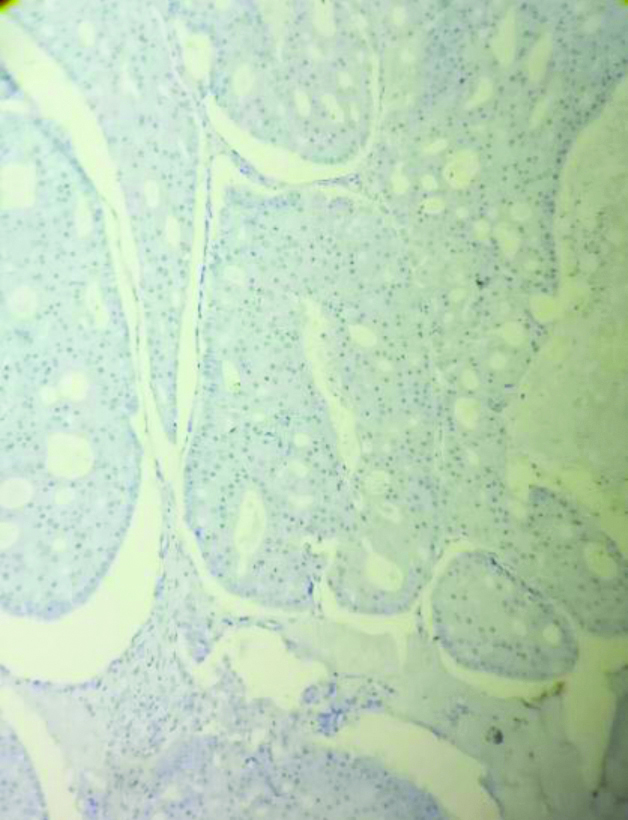
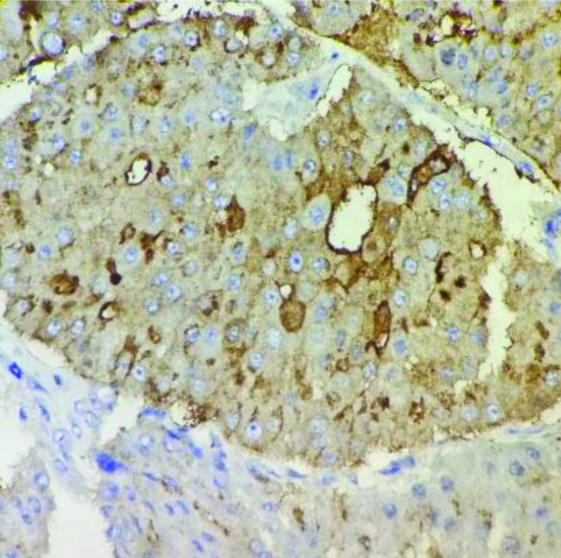
Hormone receptors like ER [Table/Fig-6], PR [Table/Fig-7], HER-2/neu [Table/Fig-8] were negative in 3 cases (60%) of AC and 213 cases (38.2%) of IDC-NST. Positivity for CK-7 was seen in all cases of AC [Table/Fig-9a]. Ki-67 was raised in all the cases (100%) of AC [Table/Fig-9b] and 279 (49.8%) IDC-NST (0.0743). p53 >10% was found in 2 cases (40%) of AC and 403 (71.96%) of IDC-NST cases (p=0.114). GCDFP-15 was positive in all the cases (100%) of AC [Table/Fig-10].
Immunohistochemical stain shows tumour cells are ER stain negative in AC (100x).
Immunohistochemical stain shows tumour cells are PR negative in AC (100 x).
Immunohistochemical stain show HER-2/neu negative tumour cells in AC (100x).
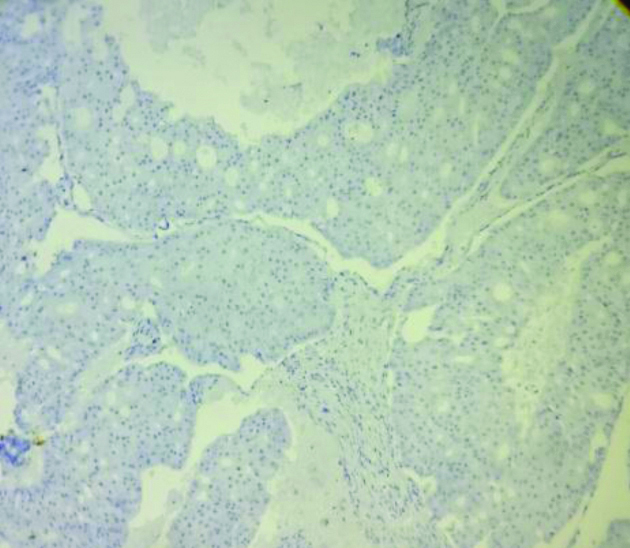
Immunohistochemical stain shows: (a) CK-7 positive in AC (400x); (b) shows Ki-67 positive in AC (400x).
Immunohistochemical stain shows GCDFP-15 positive in AC (400x).
Discussion
Apocrine cells in breast can be seen in broad spectrum of lesions ranging from simple cyst to infiltrating carcinomas. AC is an unusual type of breast carcinoma comprising <1% of breast carcinoma. IDC-NST and AC in this study comprise 83.58% and 0.74% of invasive breast carcinomas, respectively. Liao YH et al., found IDC-NST and AC in 91.6% and 1% of invasive breast carcinomas [8]. Kiyici H et al., found AC in 0.7% of invasive breast carcinomas [9]. The present study findings are comparable to these two studies. AC usually affects females in 6th and 7th decade of life [8,10]. Age of presentation for AC is higher than IDC-NST [11]. Mean age of presentation for AC and IDC-NST were 59.5 years and 50.4 years, respectively in this study. Kiyici H et al., found AC and IDC-NST in 53.9 years and 52.7 years, respectively [9]. This is comparable to the age of presentation in this study. Tumour size at presentation was ≥5 cm for both AC and IDC-NST in this study. So, most of the tumours in this study were beyond T2 stage whereas other studies found tumours in T1 and T2 stage [8,9,11]. Larger tumour size in this study may be attributable to delay in getting treatment due to illiteracy and low socioeconomic status of people in this part of India.
In present study, Japaze’s criteria was followed for diagnosis of AC from histology slides. In 2005 Japaze H et al., proposed following criteria for diagnosis of AC of breast. At least 75% of microscopic field must demonstrate the following features:
Large cells with abundant cytoplasm, usually granular.
N:C ratio > 1:2
Nuclei round, large and vesicular; may be pleomorphic.
Sharply defined cell border.
Minor criteria-
Prominent nucleoli in > 50% of fields.
Atypical cytoplasmic snouts into the lumen space [12].
In this study, 5 cases fulfilled all the above criteria. So, these cases were diagnosed as AC from histology slides. Most cases of AC and IDC-NST in this study were of grade II, which is comparable to Liao YH et al., and Zhang N et al., they also found AC of grade II in 59.8% and 46.4% cases, respectively [8,11]. Metastatic deposit in lymph node was seen in 40% of AC cases in this study. Zhang N et al., found it in 37.4% of AC [11]. Lymph node positivity in IDC-NST was twice that of AC in present study.
ER negative, PR negative and HER-2/neu negative were found in more cases of AC than IDC-NST in this study. Triple negative hormone receptor profile was seen in 3 (60%) of AC, which is higher than Tsutsumi Y and Montagna E et al., [6,13]. Ki-67 and p53 are demonstrated in most of the malignant cases in comparison to benign and borderline apocrine breast lesions [14]. So, these two markers were used for differentiating between benign and malignant apocrine lesions of breast. Moriya T et al., found Ki-67 and p53 in 75% and 46.2% of AC cases [14]. They also observed a pattern of p53 immunoreactivity in apocrine breast lesions. Intraductal and high nuclear grade AC were frequently positive for p53 compared to invasive ductal carcinoma and intermediate nuclear grade carcinoma. In present study, intermediate nuclear grade AC constituted 60% of total AC cases. This may be the reason for p53 negativity in 40% of AC cases in this study. GCDFP-15 is a 15kDa protein which is expressed in apocrine metaplasia of breast but not by normal ductal and lobular epithelium [15]. Trenkic S et al., found GCDFP-15 expression in both benign and malignant apocrine differentiation [7]. In present study, all the cases of AC were positive for GCDFP-15. However, other study found it positive in 75% of AC [16]. They also observed GCDFP-15 expression is reduced in advanced AC. GCDFP-15 positivity in all the cases of AC, in present study may be due to low histologic grade and negative nodal metastasis status in most cases. Ki-67 and p53 positivity was helpful in this study to know the malignant nature of apocrine cells which were GCDFP-15 positive. AC and oncocytic carcinoma have somewhat similar histomorphology i.e., abundant granular eosinophilic cytoplasm and low grade nuleus. Immunohistochemically apocrine cells are GCDFP-15 positive and oncocytes are GCDFP-15 negative [7]. So, GCDFP-15 may be used to differentiate AC from oncocytic carcinomas. In triple negative breast cancer, most immunomarkers of breast origin are negative except CK-7 [17]. CK-7 in present study also helped to establish the breast as cell of origin in all the AC. In hormone receptor negative AC, AR may have some therapeutic significance. AR is a steroid hormone receptor which is positive in >90% of triple negative AC. So, targeted therapy at AR in these cases may be beneficial for the patients [18].
Limitation(s)
The AR could not be tested in AC due to limited resources. So, AR status was not observed in present study. GCDFP-15 and CK-7 was done only in AC. So, these parameters could not be compared in AC and IDC-NST. Furthermore, bias in database may be there, as this was a retrospective study.
Conclusion(s)
The AC and IDC-NST have many similar clinicipathological features. However, AC is an unusual type of invasive breast carcinoma having typical cell morphology, immunohistochemical profile. Although prognostically same as IDC-NST, AC should be diagnosed as separate entity as they show different clinical behaviour with a unique response to androgens.
IDC-NST: Invasive duct carcinoma-no special type; BR: Bloom-Richardson
IDC-NST: Invasive duct carcinoma-no special type