Primitive Neuroectodermal Tumour (PNET) is a member of small round cell “Blue tumour” family. The overall incidence of PNET is <1%. PNET of kidney is uncommon and was first reported in 1975. Since the first report around 150 cases have been published in medical literature. It exhibits highly aggressive behaviour. It usually affects young adults and has a male predominance of 3:1. Only six cases of PNET of kidney have been reported in older patients and PNET of kidney as a second new primary has rarely been reported, only three cases of renal PNET with history of an earlier or synchronous primary cancer were reported in literature from the USA, Germany and China. Here, the author reported a case of renal PNET in a 48-year-old female who has been treated for right breast cancer by surgery, adjuvant chemotherapy and radiotherapy three years (July 2015) prior to diagnosis of PNET of left kidney. Upon diagnosis, patient underwent left nephrectomy. Postoperatively, patient was started on Ewing’s Family of Tumours (EFT) 2001 protocol. Patient completed induction therapy followed by radiation to tumour bed with concurrent weekly single agent vincristine and was planned for maintenance chemotherapy. After one cycle of maintenance chemotherapy, patient developed severe febrile pancytopenia and was admitted in the ICU, even after aggressive medical line of management, patient could not be revived. Although, the patient tolerated the treatment protocol for breast malignancy well and the primary was under control but after three years, with the diagnosis of a very rare second new primary PNET of left kidney which has a very aggressive nature and poor prognosis, the patient could not tolerate the multimodality treatment which was required.
Case Report
In May 2018, a 48-year-old female patient presented with symptoms of left lumbar pain and burning micturition in the Radiation Oncology Outpatient Department of a Rural Cancer Centre in Maharashtra.
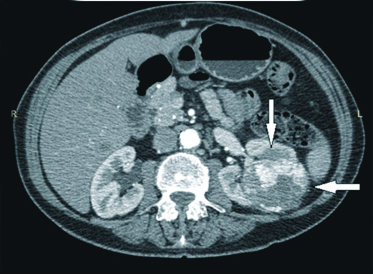
Physical examination revealed pain on percussion on left lumbar region. All laboratory investigations were unremarkable. Computerised Tomography (CT) scan revealed 9.1×8×7.4 cm mass arising from left kidney involving upper pole, fat plane between lesion and tail of pancreas lost causing displacement of splenic vessel superiorly, left renal vessel and ureter were separate with few subcentimeters sized non-necrotic lymph nodes in para-aortic region, suggesting neoplastic mass lesion [Table/Fig-1].
CT image of left lesion kidney.
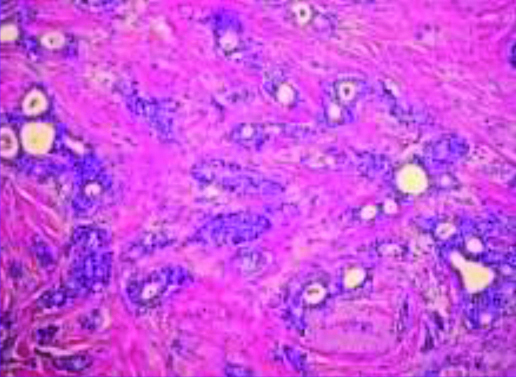
Patient’s past medical history dates back to 2014 when she was diagnosed with cancer of right breast, Infiltrating Ductal Carcinoma Grade III [Table/Fig-2] with metastasis to axillary node. She underwent right mastectomy with axillary dissection and her pathological staging was found to be pT2N2M0. Immunohistochemistry (IHC) showed oestrogen, progesterone and HER2 neu receptor negativity. Patient completed three cycles with Cyclophosphamide (500 mg/m2), Adriamycin (50 mg/m2) and 5-fluorouracil (500 mg/m2) followed by Paclitaxel (175 mg/m2) in 2015. She received radiation to chest wall and axilla, 50 GY in 25 fractions, 2 GY per fraction, five days a week and completed her treatment in July 2015. There was no evidence of disease failure locally or any distant metastases. Patient was taking regular medication for hypothyroidism and hypertension for last eight years.
GRADE III Histopathology of breast, Infiltrating ductal carcinoma (IDC).
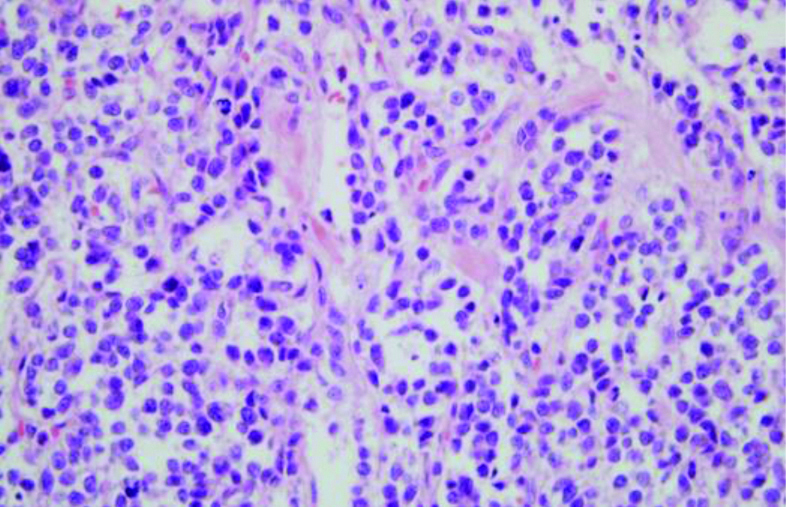
Upon diagnosis of left renal mass in May 2018, patient underwent left nephrectomy [Table/Fig-3]. Grossly, the kidney measured 12×9.5×6.5 cm and on cut-section, greyish white tumour was seen at the upper pole measuring 7.5×7×6.5 cm, predominantly in cortex with medullary involvement. Tumour was seen involving the renal sinus and pelvis without involvement of the gerota’s fascia. Microscopy sections from tumour showed features of malignant small round cell tumour, tumour cells were small round with hyperchromatic pleomorphic nuclei, prominent nucleoli and scant cytoplasm. At places, pseudo rosettes were also noted, with lymphovascular tumour invasion. Histopathology showed malignant small round cell tumour suggesting of PNET [Table/Fig-4]. Immunohistochemical staining showed strong membranous positivity for CD 99 (mic 2), diffuse positivity for vimentin and bcl2, focally for synaptophysin. Tumour cells were negative for EMA, CK7, CK20, PAX8, CD138 and desmin. Mib1 proliferative index was approximately 60%. Fluorescence In-Situ Hybridization (FISH) analysis could not be done due to patient’s financial constraints.
Histopathology of left kidney lesion, PNET.
Ewing’s family of tumours (EFT) 2001.
V: Vincristine; I: Ifosfamide; E: Etoposide; C: Cyclophosphamide; A: Adriamycin
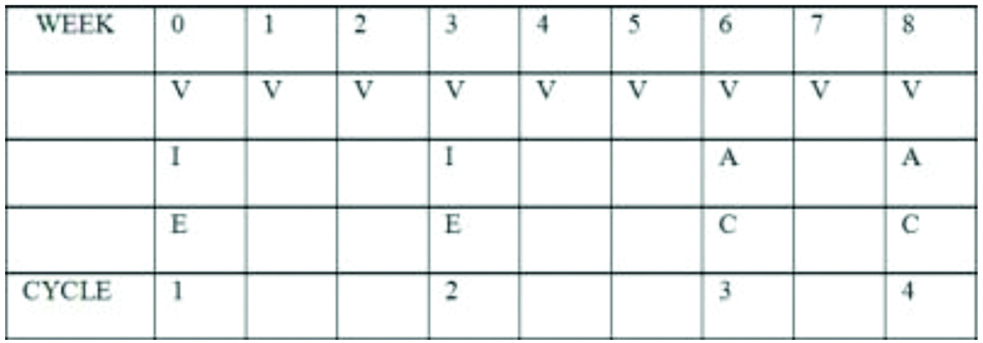
With medical oncologist consultation, patient was started on Ewing’s Family of Tumours (EFT)-2001.
The chemotherapeutic drugs used were vincristine 1.5 mg/m2 (V), adriamycin 60 mg/m2 (A), Cyclophosphamide 600 mg/m2 (C), Ifosfamide 2 g/m2 (I) and etoposide 100 mg/m2 (E), with mesna and Granulocyte Colony-Stimulating Factor (GCSF) support.
Patient completed induction therapy with EFT protocol followed by radiation to the postoperative site, 45 GY in 25 fractions, 1.8 GY per fraction (Intensity-modulated radiation therapy) with concurrent weekly single agent vincristine (last date 14/12/18). After one week of completion of radiation, patient received one-cycle maintenance chemotherapy with Vincristine, Ifosfamide and Etoposide (VIE) and GCSF support. After two weeks, patient developed severe febrile pancytopenia, (Hb-7.0 mg/dL, TLC-800, platelets-20,000 and persistent temperature of 38-39°C). Patient was shifted to the ICU and started on higher antibiotics (cefepime, tazobactam, piptaz and GCSF support) under medicine supervision, even after aggressive medical line of management the blood parameters could not be normalised and patient had to be intubated in view of persistent episodes of drop in saturation, patient expired on 6th January 2019.
Discussion
Peripheral PNET Ewing’s Sarcoma is an aggressive type of sarcoma characterised by the t(11;22)(q24;q12) translocation and overexpression of the cluster of differentiation (CD)99/MIC2 gene. PNET is predominantly considered to be a malignant bone or soft tissue tumour of children that is uncommon in older age groups (more than 30 years) [1]. The PNET of kidney is uncommon and was first reported in 1975 [2]. Since the first report around 150 cases have been published in medical literature. PNET of kidney is an aggressive disease with high metastatic potential that predominantly occurs in children and young adults (median age 28 years) and exhibits a slight male predominance [3]. Only six cases of patient more than 50 years have previously been reported. A report published by Mandal PK et al., of a 50-year-old female diagnosed with renal peripheral PNET of size 16 cm in the left kidney who underwent nephrectomy and chemotherapy with vincristine, mesna, ifosfamide and doxorubicin. Patient developed retroperitoneal lymph nodal metastasis. After following-up for one year, the patient was alive and stable [4]. In another report published by, Jimenaz RE et al., a 69-year-old female, with renal PNET of size 15 cm, was treated with nephrectomy and chemotherapy with carboplatin, VP-16, Taxol, interferon, estramustine and etoposide, developed lung and bone metastasis six months after chemotherapy. After a follow-up period of 25 months, it was discovered that the patient had expired [5]. In a similar case report published by, Koski ME et al., a 78-year-old female, developed pulmonary metastasis and expired two weeks later [6].
The PNET of kidney as a second new primary or synchronous with a different primary cancer is extremely rare, with only three reported cases from USA, Germany and China [3,4,7]. In a case report published by Eggers H et al., a 27-year-old male diagnosed with germ cell tumour of the left testicle and Ewing’s sarcoma of the right kidney simultaneously, after undergoing surgical management of the two synchronous malignancies, patient received adjuvant chemotherapy as a part of the euro-Ewing -99 study- six courses VIDE (vincristine 1.5 mg/m2 d-1, ifosfamide 3000 mg/m2 d1-3, doxorubicin 30 mg/m2 d1-3, etoposide 150 mg/m2 d1-3), patient also received high chemotherapy (treosulfan 12 g/m2 d1-3, melphalan 140 mg/m2 d1-4) with peripheral stem cell transplantation. Since 2007, the patient is in complete remission (last update 2011) [7].
In a case report similar to what we have reported in this article, a 51-year-old female, with a history of breast adenocarcinoma that was diagnosed and treated five years prior to the diagnosis of renal PNET. The patient underwent nephrectomy with the tumour size of 11 cm. The patient declined adjuvant chemotherapy but accepted traditional chinese medicine (six ingredient Rehmania pill). The 8th month follow-up confirmed that the patient was alive [8].
The presenting symptoms and radiographic features of renal PNET are non-specific. Therefore, diagnosis of peripheral PNET is mainly based on morphological, immunohistochemical and genetic analysis [9]. Histologically, PNET consists of sheets and nests of primitive small round blue cells and presence of homer-wright rosettes [10]. PNET of kidney must be differentiated from other small round cell tumours such as wilms, malignant lymphoma, small cell carcinoma, rhabdomyosarcoma, desmoplastic small round cell tumour. Strong membrane positivity for CD99 and FLI1 are characteristic features of PNET. However, they are not definite features, therefore an immunohistochemical panel consisting CD99 and Friend Leukemia Intergartion transcription factor (FLI1) and other relevant differential markers are recommended [11].
Treatment strategies of renal PNET are multimodal including surgery, adjuvant chemotherapy and radiotherapy [2]. Owing to the difficulty in preoperative diagnosis, neoadjuvant chemotherapy before surgery is not applicable. Therefore, majority of patients undergo radical nephrectomy as the initial treatment. Since PNET biologically behaves like Ewing’s sarcoma, it should be treated with a combination of radical nephrectomy and chemotherapy, which includes vincristine, doxorubicin, cyclophosphamide, etoposide and ifosfamide. In cases where there is an incomplete resection and positive margin or recurrence of the tumour, radiation is recommended. Owing to high aggressiveness and strong tendency of local recurrence, the overall prognosis of renal PNET is poor. A long-term survival (7.5 years) of 45-60% was noted for peripheral PNET patients. Central PNET patient survival was much shorter with a three-year survival of only 33%.
Conclusion(s)
The PNET of kidney is very rare and even rarer in older females or in patients, who have received treatment for other type of cancer in the past. The current study presents a case of 48-year-old female, with left renal PNET as a second new primary following breast infiltrating ductal carcinoma. Written informed consent was taken from the patient’s son and father.
Declaration: The abstract of this article was presented as a poster in the 41st annual conference of the Association of Radiation Oncologists of India (AROICON 2019) 28th November-1st December, Ahmedabad, Gujarat, India.
[1]. Howe HL, Wu X, Ries LAG, Cokkinides V, Ahmed F, Jemal A, Annual report to the nation on the status of cancer, 1975-2003, featuring cancer among U.S. Hispanic/Latino populationsCancer 2006 7(8):1711-42.10.1002/cncr.2219316958083 [Google Scholar] [CrossRef] [PubMed]
[2]. Seemayer TA, Thelmo WL, Bolande RP, Wiglesworth FW, Peripheral neuroectodermal tumoursPerspect Pediatr Pathol 1975 2:151-72. [Google Scholar]
[3]. Risi E, Iacovelli R, Altavilla A, Alesini D, Palazzo A, Mosillo C, Clinical and pathological features of primary neuroectodermal tumour/Ewing sarcoma of the kidneyUrology 2013 2(2):382-86.10.1016/j.urology.2013.04.01523800653 [Google Scholar] [CrossRef] [PubMed]
[4]. Mandal PK, Mukherjee S, Roy S, Bhattacharyya NK, PNET of kidney: Report of four casesIndian J Med Paediatr Oncol 2012 3(2):130-33.10.4103/0971-5851.9975422988357 [Google Scholar] [CrossRef] [PubMed]
[5]. Jimenez RE, Folpe AL, Lapham RL, Ro JY, O’Shea PA, Weiss SW, Primary Ewing’s sarcoma/primitive neuroectodermal tumour of the kidney: A clinicopathologic and immunohistochemical analysis of 11 casesAm J Surg Pathol 2002 26:320-27.10.1097/00000478-200203000-0000511859203 [Google Scholar] [CrossRef] [PubMed]
[6]. Koski ME, Tedesco JM, Clark PE, Renal peripheral neuroectodermal tumour presenting at age 78: Case reportScientific World Journal 2008 8:830-34.10.1100/tsw.2008.10918758660 [Google Scholar] [CrossRef] [PubMed]
[7]. Eggers H, Waalkes S, von Klot C, Tränkenschuh W, Merseburger AS, Herrmann TR, [Ewing’s sarcoma of the kidneys with simultaneous seminoma]Urologe A 2011 50:205-07.10.1007/s00120-010-2488-021312084 [Google Scholar] [CrossRef] [PubMed]
[8]. Zhong J, Chen NI, Chen X, Gong J, Nie L, Xu M, Peripheral primitive neuroectodermal tumour of the kidney in a 51-year-old female following breast cancer: A case report and review of the literatureOncology Letters 2015 9:108-12.10.3892/ol.2014.269525435942 [Google Scholar] [CrossRef] [PubMed]
[9]. Maccioni F, Della Rocca C, Salvi PF, Manicone AM, Ascarelli A, Longo F, Malignant peripheral neuroectodermal tumour (MPNET) of the kidneyAbdom Imaging 2000 25:103-06.10.1007/s00261991002110652933 [Google Scholar] [CrossRef] [PubMed]
[10]. Romero FR, Rais-Bahrami S, Muntener M, Permpongkosol S, Fine SW, Paidas CN, Metastatic primitive neuroectodermal tumour to the kidneyUrol Int 2007 78(3):286-88.10.1159/00009935517406144 [Google Scholar] [CrossRef] [PubMed]
[11]. Saxena R, Sait S, Mhawech-Fauceglia P, Ewing sarcoma/primitive neuroectodermal tumour of the kidney: A case reportDiagnosed by immunohistochemistry and molecular analysisAnn Diagn Pathol 2006 10(6):363-66.10.1016/j.anndiagpath.2005.11.00117126256 [Google Scholar] [CrossRef] [PubMed]