Introduction
Dental caries is a pandemic disease caused by Streptococcus mutans. Numerous preventive strategies have been developed to prevent dental caries with modern medicines. Recently, there has been a shift from use of modern medicines to herbal ayurvedic preparations which are easily available and cause least possible side effects but have not been tested against S. mutans.
Aim
To evaluate antimicrobial value of Pudina, Tulsi and Curry leaves against S. mutans by modified direct contact test.
Materials and Methods
This in vitro study used finely powdered form of Pudina, Tulsi and Curry leaves extracts (Hakim Chi Chi Pharmacy, Surat, India) without any added preservatives. The test microorganism S. mutans was isolated from human saliva using mitis salivarius agar. The Minimum Inhibition Concentration (MIC) was determined using agar well diffusion for Group 1 (Tulsi), Group 2 (Pudina), Group 3 (Curry leaves) and Group 4 (Chlorhexidine). Using this MIC, modified Direct Contact Test was performed for specific evaluation of the antimicrobial efficacy of each product. Chlorhexidine (0.2%) was used as a positive control for the comparison of each product. The results of modified direct contact test were subjected to ANOVA Test and Tukey’s Test using SPSS software version 23.0 which showed that when compared against chlorhexidine, the antimicrobial efficacy against S. mutans in decreasing order was of curry leaves, tulsi and pudina and the result of the study was significant.
Results
Curry leaves showed higher antimicrobial efficacy than tulsi and pudina when compared against chlorhexidine (control) and the results were statistically significant (p<0.05).
Conclusion
Curry leaf extract shows promising antimicrobial property against S. mutans and can be recommended for caries control with further investigations.
Introduction
Dental diseases are recognised as one of the major public health problems worldwide and are the most common affliction of mankind [1]. The incidence of dental caries is steadily increasing in underdeveloped and developing countries. Dental caries is a microbial infectious disease majorly caused by pioneer bacteria S. mutans and Lactobacillus Acidophilus leading to degradation of the tooth structure. The other factors aiding to the dental caries are carbohydrates, acids, dental plaque and host factors. Various strategies have been formulated ranging from preventive to restorative to manage dental caries. Restorative treatment is expensive and not so easily approachable to the poor, hence there is a vital need to promote traditional preventive measures that are acceptable, easily available and cost effective [2]. Various methods are used for plaque control, which are mechanical or chemical and either self-applied or professionally applied to reduce microbial load of the oral cavity. Chlorhexidine (0.2%) has been frequently used for its antibacterial and antiplaque properties but is associated with brownish discolouration of teeth and loss of taste sensation as its side effect [3].
In recent times, a shift from use of modern medicines to herbal ayurvedic preparations has been observed as these preparations are easily available and cause least possible side effects. Use of herbs and their extracts has become the key source for medicine for 80% of the global population as reported by World Health Organisation [4].
Many plants and plant substances like tulsi, pudina, neem, garlic, cinnamon, eucalyptus, rosemary and clove have been demonstrated to have antimicrobial properties and are effective against S. mutans [5].
Pudina (Menthaarvenis) is an important culinary plant with immense medical use. It has antibacterial and antifebrile effects. Essential oil and menthol can treat indigestion, fever, throat and sinus ailments, acne and burn. Menthol, the active constituent present in pudina, perhaps is largely responsible for its therapeutic potential [6]. It is used to treat liver and spleen diseases, asthma and jaundice. The essential oil is antiseptic, antibacterial, stimulant and diuretic [5].
The curry leaf plant (Murrayakoenigii) is grown for its aromatic foliage which is utilised as an ingredient in Indian gastronomy. The leaves are recognised for its antioxidant, antimicrobial, antidiabetic, antiinflammatory and hepatoprotective properties [5].
Tulsi (Ocimumtenuiflorum) has its role in traditional medicine from time immemorial with its effective antidiabetic, antioxidant, antibacterial, analgesic and disinfectant properties. It is beneficial in treating tooth ache, halitosis, pyorrhea, respiratory disorders, fever, eye care and headache [5].
In view of very little evidence [1,7-9], regarding the comparison between these three products against S. mutans, we made the choice to evaluate the antimicrobial efficacy of pudina (Menthaarvenis), tulsi (Ocimumtenuiflorum) and curry leaves (Murrayakoenigii).
Routinely, an Agar Diffusion Test (ADT) is used to determine the antimicrobial activity of different materials. However, because of its limitations like its dependency on diffusion and physical properties of tested materials, it is no longer recommended to be used for this purpose [10,11]. The modified direct contact test is both qualitative and reproducible assay and enables to calculate directly after each contact time, the precise amount of viable bacteria present [12]. As compared to the direct contact test a modified direct contact test enables measurement of the bactericidal effect instead of bacteriostatic effect of the materials. Thus, this study strived to evaluate the antimicrobial efficacy of pudina (Menthaarvenis), tulsi (Ocimumtenuiflorum) and curry leaves (Murrayakoenigii) extracts against S. mutans by modified direct contact test as the literature is scanty for the comparison of these three materials using this newer bacterial testing methodology.
Materials and Methods
This in vitro study was carried out at School of Dental Sciences, KIMSDU, Karad in November 2015 after due permission from the Institutional Ethics Committee (Ref No: KIMSDU/ICMR/STS/2015). The study duration was six months and was carried out between January and June of 2016.
Herbal Extracts
A pilot study prior to this investigation was carried out to find out the feasibility of the study with the below mentioned extracts. Based on the Alpha type I error set at 0.05 and power of 90%, a sample size of 15 was determined to be adequate for the current study. Finely, powdered form of 50 grams each of pudina (Menthaarvenis), tulsi (Ocimumtenuiflorum) and curry leaves (Murrayakoenigii) were obtained from an Ayurvedic Pharmacy (Hakim ChiChi Pharmacy, Surat, India) without any added preservatives.
The powdered samples were divided into four groups as follows (n=15)
Group 1- Pudina extract (HakimChiChi Pharmacy, Surat, India)
Group 2- Tulsi extract (HakimChiChi Pharmacy, Surat, India)
Group 3- Curry leaves extract (HakimChiChi Pharmacy, Surat, India)
Group 4- Chlorhexidine 0.2% (Control) (Clohex Mouth Wash, Dr Reddy’s Laboratories Ltd., India)
Microorganisms
The saliva samples used for this study were collected from the five patient’s mouth diagnosed with at least three active carious lesions after a written informed consent was taken. Whole, stimulated saliva by spitting method was collected by one of the investigators by asking the patient to bite on a standard piece of paraffin wax. The saliva was transferred to a sterile screw capped tube and the test microorganism S. mutans were isolated and subcultured using a specific media, mitis salivarius agar, bacitracin (HiMedia Laboratory Pvt., Ltd., Bombay India).
Sample Preparation
The extract of herbal products was prepared by dissolving 1 μg of the powder in 1 μL of distilled water as per the protocol given by Prashant GM et al., [1]. Once the extract was prepared, dilutions were prepared to achieve a range of 500% to 5% (V/V). To obtain 500% (V/V), 50 gm of powder was added to 10 mL of distilled water. Minimum Inhibition Concentration (MIC) was then determined using agar well diffusion.
Minimum Inhibition Concentration (MIC) of Extract
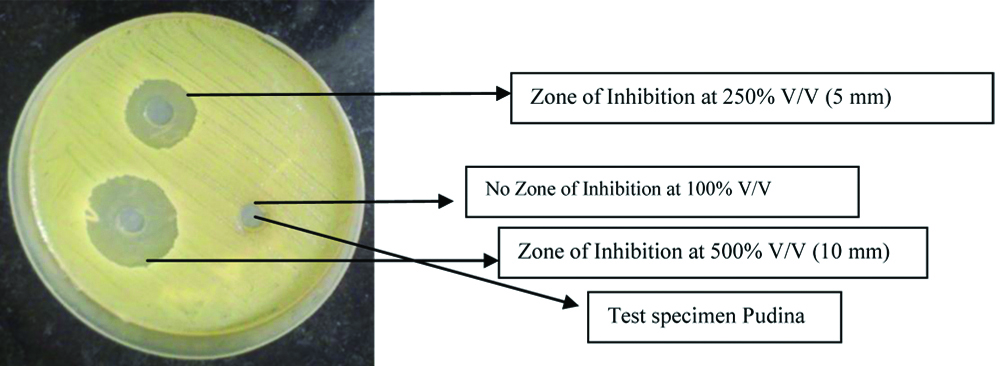
The MIC of the extracts was determined using the method proposed by Thongston C et al., [13]. Fifteen samples (n=15) for each group were prepared and an Agar Well Diffusion Technique was used to determine the MIC for all the groups. The bacterial isolates seeded on Mueller Hinton agar plates were fed with a 0.5 mL volume of each solution using aseptic technique and were incubated at 37°C for 24 hours. The least concentration of extracts showing clear zone of inhibition in millimeters (mm) was measured using vernier calipers (Aerospace Digimatic Vernier Caliper 150 mm/6 inch, India) and was considered as the MIC [Table/Fig-1,2]. Once the MIC was determined; the antimicrobial efficacy was then tested using the modified direct contact test.
Minimum Inhibition Concentration (MIC) showing zone of inhibition.
| Variables | Zone of Inhibition (in millimetres) |
|---|
| Standard (Control) | Serial Dilution percentage (V/V) |
|---|
| 5% (V/V) | 25% (V/V) | 50% (V/V) | 100% (V/V) | 250% (V/V) | 500% (V/V) |
|---|
| Group 1: Pudina | | No inhibition zone | No inhibition zone | No inhibition zone | No inhibition zone | 5 mm | 10 mm |
| Group 2: Tulsi | | No inhibition zone | No inhibition zone | No inhibition zone | No inhibition zone | No inhibition zone | 9 mm |
| Group 3: Curry leaves | | No inhibition zone | No inhibition zone | No inhibition zone | No inhibition zone | No inhibition zone | 7 mm |
| Group 4: Chlorhexidine (0.2%) | 9 mm | | | | | | |
Zone of Inhibition for Pudina group depiciting 500%, 250%, 100% (V/V).
Modified Direct Contact Test
The antibacterial property (cfu/mL) of the extracts was determined using the protocol set by Zhang H et al., [11]. A 96 well microtitre plate was held vertically and an area of fixed size on the side wall of the wells was coated with an equal amount (100 μL) of each extract (Group 1, Group 2, Group 3) with an applicator tip. A 10 μL of bacterial suspension {3×108 cfu/mL which contains 3×106 bacteria determined using a spectrophotometer (UV 2550 Shimadzu, Kyoto, Japan)} was carefully placed on the surface of each extract. Bacterial suspensions with 0.2% chlorhexidine served as a control. After incubation in 100% humidity at 37°C for two minutes, 240 μL of Tryptic Soya Broth (TSB) was added to each well. After gently mixing with pipette for one minute, the bacterial suspension from each well was transferred and serially diluted in TSB. The viability of bacteria was appraised by culturing aliquots of 20 μL onto Tryptic Soya Agar (TSA) plate subsequent to 10-fold sequential dilutions. The colony forming units/mL was calculated using the formula cfu/mL=(no. of colonies×dilution factor)/volume of culture plate after 24 hours incubation at 37°C. All the tests were performed three times using the same titre plate to reduce the manual error. Triplicate testing methodology was used.
Statistical Analysis
The significance level (p-value) was set to <0.05. All tests were performed using the software program SPSS for Windows, version 23.0 (IBM, NY, USA). The results were analysed using ANOVA test followed by Tukey post-hoc test for intergroup comparison.
Results
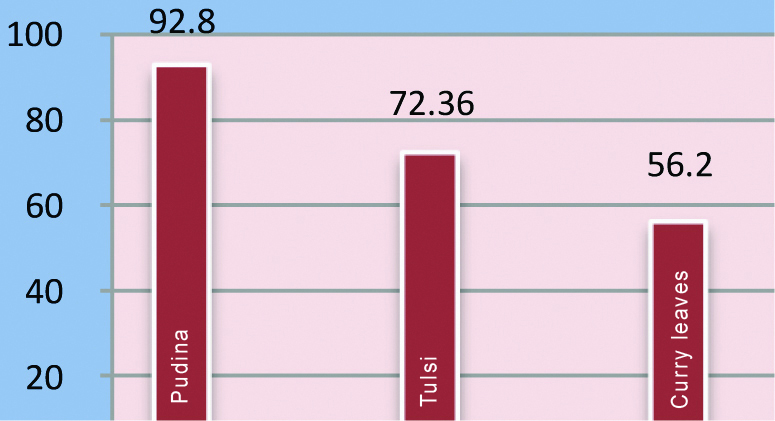
The zone of inhibition for each group (in mm) attained using agar well diffusion of different groups are listed in the [Table/Fig-1] and depicted in [Table/Fig-2]. The mean, standard deviation and standard error for antibacterial property of extracts using modified direct contact test in millimeters is presented in [Table/Fig-3]. The growth of S. mutans was measured and analysed. The data was subjected to ANOVA and Tukey’s post-hoc test.
Mean, Standard Deviation and standard error for antibacterial property of herbal extracts using modified direct contact test.
| Extract | Mean (in mm) | Standard deviation (in mm) | Standard error (in mm) |
|---|
| Group 1: Pudina | 92.8 | 11.2 | 2.2 |
| Group 2: Tulsi | 72.36 | 12.3 | 2.45 |
| Group 3: Curry leaves | 56.2 | 14.2 | 2.65 |
| Group 4: Chlorhexidine (0.2%) | 22.5 | 2.4 | 1.23 |
Amongst the three study groups curry leaves had the lowest mean bacterial count (cfu/mL) [Table/Fig-4]. ANOVA test [Table/Fig-5] revealed a statistically significant difference (p<0.0003) among the groups compared. Tukey’s post-hoc test [Table/Fig-6] was employed for intergroup comparison. This test revealed a statistically significant difference between pudina and curry leaves (p=0.0151), pudina and chlorhexidine (p=0.0002), tulsi and chlorhexidine (p=0.0024) curry leaves and chlorhexidine (p=0.0233) [Table/Fig-6].
Graphical representation of the mean growth of streptococcus mutants.
Comparison of all the four groups (pudina, tulsi, curry leaves and chlorhexidine) using the ANOVA test to check the antibacterial efficacy of the products against S. mutans.
| Sum of squares | Df | Variance | F | p-value |
|---|
| Between | 7936.7241 | 3 | 2645.5747 | 21.8584 | 0.0003* |
| Within | 968.260 | 8 | 121.0325 | | |
| Total | 8904.9841 | 11 | | | |
The Tukey’s post-hoc test showing results regarding the antibacterial activity against S. mutants among all groups.
| Comparison | Difference | 95% CI | p (<0.05) |
|---|
| Pudina vs. Tulsi | -20.4400 | -49.2057 to 8.3257 | 0.1831 |
| Pudina vs. Curry leaves | -36.6000 | -65.3657 to -7.8343 | 0.0151* |
| Pudina vs. Chlorhexidine | -70.3000 | -99.0657 to -41.5343 | 0.0002* |
| Tulsi vs. Curry leaves | -16.1600 | -44.9257 to 12.6057 | 0.3401 |
| Tulsi vs. Chlorhexidine | -49.8600 | -78.6257 to -21.0943 | 0.0024* |
| Curry leaves vs. Chlorhexidine | -33.7000 | -62.4657 to -4.9343 | 0.0233* |
Discussion
In recent years, the traditional method of using herbal products in treatment of various diseases has resurfaced and there has been a rise in its use. The reason behind this is the easy availability of herbal products, cost-effectiveness, low toxicity, increased shelf life and lack of microbial resistance. Thus, spices and other herbal products which are normal ingredients of our routine food preparations can provide protection against bacterial pathogens; serving as therapeutic agents containing antibiotics and other medicinal compounds [14,15].
Chlorhexidine (0.2%) was chosen as the control as it is known to have a potent antibacterial effect against S. mutans. But long-term use of chlorhexidine has side effects like staining of teeth, loss of taste and sometimes stenosis of the parotid duct [3]. Unlike it, herbal products are known to have least side effects with an advantage of affordability to all classes of people. Pudina, tulsi and curry leaves are known to have antibacterial properties. But their effect on S. mutans has been assessed using traditional methods with doubtful accuracy. Hence, we have made an attempt to compare the antibacterial properties of pudina, tulsi, curry leaves and chlorhexidine against S. mutans using modified direct contact test for accurate and better evaluation of the antimicrobial efficacy. The powdered herbal products were serially diluted to obtain the MIC against S. mutans. Distilled water was chosen as the solvent instead of methanol to check for the pure antibacterial effect without the properties of methanol overshadowing the results. Bacteriological testing was performed by modified direct contact test as demonstrated by Zhang H et al., [11].
The result of the modified direct contact test showed that the least mean growth of S. mutans was seen with curry leaf, when compared to the control chlorhexidine. The products in their decreasing order of mean growth of S. mutans were chlorhexidine, curry leaves, tulsi and pudina. The anti bacterial properties of curry leaf can be attributed to its unique compounds carbazole derivatives, murrayanol and girinimbine present in the leaves [15-17].
The statistical analysis using ANOVA test (p<0.05) showed significant difference (p=0.0003) between the antibacterial efficacy of the groups against S. mutans. When compared with the control group chlorhexidine (0.2%), curry leaves were the most effective of the three products and this was in accordance to the study done by Chandra Shekar BR et al., [7]. Prabhakar AR and Murlikrishnan NL, conducted a study comparing curry leaves, garlic and tea tree oil against S. mutans and found all three herbal products had a significant antibacterial property against the tested organism [18]. When subjected to the Tukey’s post-hoc test, there was a significant difference between the antimicrobial efficacy of pudina and curry leaves (p=0.0151), pudina and chlorhexidine (p=0.0002), curry leaves and chlorhexidine (p=0.0233); and tulsi and chlorhexidine (p=0.0024). Chaudhary NJ et al., conducted a study comparing the antimicrobial effect of pudina and chlorhexidine and concluded that though pudina was effective against S. mutans; its efficacy was less compared to chlorhexidine [8]. Agarwal P et al., conducted a study evaluating the antimicrobial properties of tulsi and chlorhexidine, results of which concluded chlorhexidine had better action against S. mutans than Tulsi [9]. Thus, the results of this study are in agreement with the previous studies [7-9] where both pudina and tulsi had less efficacy against S. mutans as compared to chlorhexidine (0.2%) and curry leaves were potent against cariogenic bacteria.
Limitation(s)
This investigation was conducted in vitro with the extracts of tulsi, pudina and curry leaves. The duration of the contact of such extracts with the microorganisms in the oral cavity in-vivo is not clear; hence, further in-vivo studies are required to obtain clinical results comparing these products utilising a multibacterial biofilm.
Conclusion(s)
Under the limitations of this study, curry leaves had better antimicrobial efficacy compared to pudina and tulsi. With the changing trends in dental caries initiation and progression the use of various preventive strategies have been modified and the inclination towards the use of safer, cost-effective herbal products has increased.
[1]. Prashant GM, Chandu GN, Murulikrishna KS, Shafiulla MD, The effect of mango and neem extracts on four organisms causing dental caries: Streptococcus mutans, streptococcus salivavius, streptococcus mitis and streptococcus sanguis: An invitro studyIndian J Dent Res 2007 18(4):148-51.10.4103/0970-9290.3582217938488 [Google Scholar] [CrossRef] [PubMed]
[2]. Abascal K, Yarnell E, Herbs and drug resistanceAlternative and Complementary Therapies 2002 8(4):237-41.10.1089/107628002320351370 [Google Scholar] [CrossRef]
[3]. Fardal O, Turnbull RS, A review of the literature on use of chlorhexidine in dentistryJ Am Dent Assoc 1986 112(6):863-69.10.14219/jada.archive.1986.01182940282 [Google Scholar] [CrossRef] [PubMed]
[4]. World Health OrganizationSummary of WHO guidelines for the assessment of herbal medicinesHerbal Gram 1993 28:13-14. [Google Scholar]
[5]. Liu Q, Meng X, Li Y, Zhao CN, Tang GY, Li HB, Antibacterial and antifungal activities of spicesInt J Mol Sci 2017 18(6):128310.3390/ijms1806128328621716 [Google Scholar] [CrossRef] [PubMed]
[6]. Singh S, More PK, Mohan SM, Curry leaves (Murraya koenigii Linn. Sprengal)-A mircale plantJ Sci Res 2014 4(1):46-52. [Google Scholar]
[7]. Chandra Shekar BR, Nagarajappa R, Jain R, Singh R, Thakur R, Shekar S, Antimicrobial efficacy of acacia nilotica, murrayakoenigii (l.) sprengel, eucalyptus hybrid, psidiumguajava extracts and their combination on streptococcus mutans and lactobacillus acidophilusDent Res J (Isfahan) 2016 13(2):168-73.10.4103/1735-3327.17820627076832 [Google Scholar] [CrossRef] [PubMed]
[8]. Chaudhary NJ, Krishnan CGA, Thanveer K, Shah H, Anti-microbial effect of Pudina extract on streptococcus mutans: Invitro studyJournal of International Oral Health 2012 4(3):45-49. [Google Scholar]
[9]. Agarwal P, Murlikrishnan NL, Evaluation of antimicrobial activity of various concentrations of Tulsi (Ocimum sanctum) extract against S. mutans. An invitro studyIndian J Dent Res 2010 21(3):357-59.10.4103/0970-9290.7080020930344 [Google Scholar] [CrossRef] [PubMed]
[10]. Haapasalo M, Qian W, Irrigants and intracanal medicaments.in: Ingle JI. Bakland LKBaumgartner JC Ingle’s endodontics 2008 6th edHamilton, ON, CanadaBC Decker Inc:992-1011. [Google Scholar]
[11]. Zhang H, Shen Y, Ruse ND, Haapasalo M, Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalisJ Endod 2009 35(7):1051-55.10.1016/j.joen.2009.04.02219567333 [Google Scholar] [CrossRef] [PubMed]
[12]. Almas K, Al-Lafi TR, The natural toothbrushWorld Health Forum 1995 16:206-10. [Google Scholar]
[13]. Thongston C, Davidson PM, Mahakarnchanakul W, Weiss J, Antimicrobial activity of ultrasound-assisted solvent-extracted spicesLetter in Applied Microbiology 2004 39(5):401-06.10.1111/j.1472-765X.2004.01605.x15482429 [Google Scholar] [CrossRef] [PubMed]
[14]. Al-lafi T, Ababneh H, The effect of extract of the miswak (chewing sticks) used in Jordan and The Middle East on oral bacteriaInt Dent J 1995 45(3):218-22. [Google Scholar]
[15]. Akram M, Uzair M, Malik NS, Mahmood A, Sarwer N, Madni A, MenthaArvenis Linn.: A review articleJ Med Plant Res 2011 5(18):4499-503. [Google Scholar]
[16]. Vats M, Singh H, Sardana S, Phytochemical screening and antimicrobial activity of roots of Murrayakoenigii (Linn.) Spreng. (Rutaceae)Braz J Microbiol 2011 42:1569-73.10.1590/S1517-8382201100040004424031791 [Google Scholar] [CrossRef] [PubMed]
[17]. Saini SC, Reddy GB, A review on curry leaves (Murrayakoenigii): Versatile multi potential medicinal plantAm J Phytomed Clin Ther 2015 3(4):363-68. [Google Scholar]
[18]. Prabhakar AR, Ahuja V, Basappa N, Effect of curry leaves, garlic and tea tree oil on streptococcus mutans and lactobacilli in children: A Clinical and Microbiological StudyPesqui Bras Odontopediatria Cl Integr 2009 9(3):259-63.10.4034/1519.0501.2009.0093.0002 [Google Scholar] [CrossRef]