GISTs are most common mesenchymal tumours in the GIT has been frequently studied and published in the western literature as well as in India [1,2]. They occur more frequently in stomach (60%), small intestine (25%) and less commonly in other gastrointestinal regions (oesophagus, colon and rectum) [3,4]. They can also rarely arise outside the GIT from retroperitoneum, mesentery and omentum called as EGISTs [2-5]. Earlier, GIST was included along with leiomyomas, leiomyosarcomas and leiomyoblastomas, however, now it is being considered as separate entity. Mazur MT and Clark HB were the first to coin the term GIST and proved that these non-epithelial tumours lacked the immunohistochemical features of schwann cells and ultrastructural features of smooth muscle cells [6]. Now, they have been proved to be arising from the smooth muscle pacemaker interstitial cells of Cajal, these cells are involved in gut motility and peristaltic movements [2,4,5].
The pathogenesis of GISTs was explained by Hirota S et al., observing the c-KIT (cytoplasmic tyrosine kinase) or CD117 (cluster of differentiation) mutations [7], while subsequently, Heinrich MC et al., found mutations of Platelet-Derived Growth Factor Receptor A (PDGFRA) in GISTs without c-KIT gene mutations [8]. c-KIT (CD 117) and PDGFRA genes encode for similarly named highly homologous receptor, tyrosine kinase proteins and the KIT gene encodes the KIT protein, which is the transmembrane receptor for the cytokine known as Stem Cell Factor (SCF). Whereas in 10-15% of cases these mutations are not seen, they are referred as wild type GISTs [1,4]. GISTs and EGISTs were usually misdiagnosed due to lack of characteristic symptoms and a low incidence rate. Hence, these lesions become the interest of pathologists and clinicians because of the treatable nature of disease in the initial stage and physician’s ability to relieve symptoms at an early stage. This study was done to analyse the clinical features, histopathological and immunohistochemical characteristic features of GISTs and EGISTs.
Materials and Methods
This retrospective observational (cohort) study comprised of 12 cases of GISTs diagnosed over a period of two years from January 2018 to December 2019, in Department of Pathology, Navodaya Medical College and Hospital, Raichur, Karnataka. All cases of resected biopsy specimens both gastrointestinal and extra gastrointestinal were included in the study and documented. Detailed clinical information was recorded from the case records; this included the age and sex of the patients, duration of illness, site of biopsy, symptoms, complete blood counts and radiological findings. An ethical clearance was obtained from the ethical committee of the institute.
Sections stained with Haematoxylin and Eosin (H and E) were used to evaluate growth pattern, morphology of the cells and their relative number, mitotic figures per 50 random High Power Field (HPF) and also their capsular and extra-capsular presence. Subsequently, 5-μm thick sections were cut and taken on poly-L-lysin coated slides.
Immunohistochemistry (IHC) staining was performed by using streptavidin biotin conjugate immunoperoxidase method. The slides were first treated with 0.1M citrate buffer (pH 6.0) by microwave method for antigen retrieval and incubated overnight at 4°C. Immunohistochemical detection was performed with primary antibody CD117 (45, Dako, 1:2000). Diaminobenzidine was used as the chromogen and all the slides were counter stained with haematoxylin and appropriate controls were used. The CD117, immunostaining staining patterns noted were cytoplasmic, nuclear and membranous. The IHC scoring was done based on percentage of cells and intensity of staining as positive, if cells with more than 10% positivity and based on that, were further classified into weakly positive (10%-25%), moderately positive (26%-75%) and strongly positive (>75%) [9].
Statistical Analysis
All the patients’ data were collected and entered into the excel sheets. Statistical analysis was carried out by using Statistical Package for the Social Sciences (SPSS) version 15.0 software and descriptive statistical methods like mean, median and percentage were calculated.
Results
In total, 12 cases of GIST were included in this study. There was a wide age distribution of patients ranging from 4 to 70 years with mean age of 50.6 years. GISTs had preponderance in males as compared to females, with male to female sex ratio being 2:1.
The majority were located in large intestine 4 (33.3%) cases, followed by stomach 3 (25%) cases and jejuno-ileal junction 2 (16.6%) cases. Among three cases (25%) of extra-gastrointestinal category, two cases were in retroperitoneum and one from gastric mesentery.
The clinical presentation according to site of tumour, for gastric GISTs upper GI bleeding, pain abdomen, lump and vomiting were more common, whereas in jejunoileal and transverse colon GISTs, pain abdomen, abdominal lump, lower GI bleeding and diarrhea were common, and rectosigmoid GISTs (2 cases and one with metastasis to liver) were associated with pain abdomen, bleeding per rectum and constipation. Retroperitoneal extraintestinal GIST (1 case) was presented with pain in the right hip due to secondaries and gastric serosal extraintestinal GIST (1 case) was associated with gastric adenocarcinoma with history lump and pain abdomen [Table/Fig-1].
Clinical features based on the location.
| Gastrointestinal stromal tumours |
|---|
| Location | Symptoms | No. (Percentage) |
|---|
| Stomach (n=3) | UGI bleeding | 3 (100%) |
| Abdominal pain | 3 (100%) |
| Abdominal mass | 3 (100%) |
| Anorexia/Nausea/Vomiting | 2 (66.6) |
| Jejunoileal (n=2) | LGI bleeding | 2 (100%) |
| Abdominal pain | 2 (100%) |
| Abdominal mass | 2 (100%) |
| Transverse colon (n=2) | Abdominal mass | 2 (100%) |
| Rectosigmoid (n=2) | LGI bleeding | 2 (100%) |
| Abdominal pain | 1 (50%) |
| Abdominal mass | 2 (100%) |
| Constipation | 2 (100%) |
| Extra gastrointestinal stromal tumours |
| Retroperitoneum (n=2) | Abdominal pain | 1 (50%) |
| Abdominal mass | 1 (50%) |
| Mesentery (n=1) | Abdominal pain | 1 (100%) |
| Abdominal mass | 1 (100%) |
UGI: Upper gastrointestina; LGI: Lower gastrointestinal
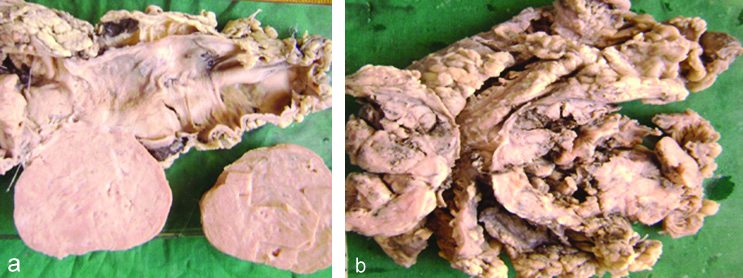
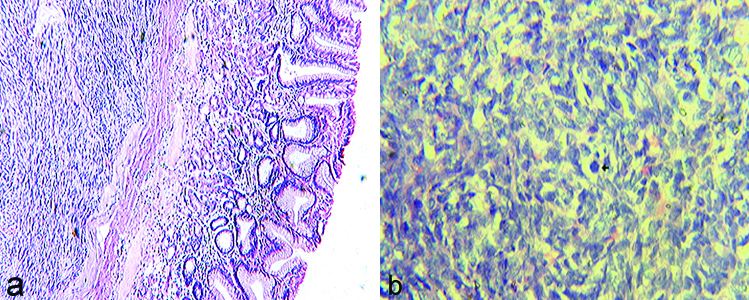
Grossly, the size of tumours ranges from 2×4 cm to 10×16 cm and cut surfaces varied from grey-white to greyish-brown with areas of necrosis and haemorrhage [Table/Fig-2a,b]. Microscopically majority of the cases 8 (70%) showed spindle cell morphology, 2 (15%) cases showed epithelioid cell type and 2 (15%) were mixed type [Table/Fig-3a,b]. The 7 (58.3%) cases were diagnosed as benign GISTs and other 5 (41.6%) were malignant [Table/Fig-4]. The malignant potential was diagnosed based on the tumour size >2 cm and number of mitotic figures >5/50 HPFs. CT scan was done in 2 malignant GISTs which confirmed metastasis.
a) Rectosigmoid tumour with flattened mucosa. b) Transverse colon with tumour arising from the wall.
a) Benign GIST showing well circumscribed lesion with spindle shaped cells in short fascicles (H and E x100). b) Malignant GIST showing spindle cells arranged in whorls with increased mitotic activity (H and E x400).
Demographic, Histopathological characters and Immunotyping.
| Sl. No. | Age | Sex | Site | Size of lesion | Type of cells | HP diagnosis | Metastasis | CD 117 |
|---|
| Gastrointestinal stromal tumours |
| 1. | 60 | M | Stomach | 5×4×3 cm | Epithelioid | Benign GIST | - | + |
| 2. | 58 | M | Stomach | 10×8×6 cm | Spindle | Malignant GIST | Iliac crest bone | - |
| 3. | 65 | M | Transverse colon | 8×4×4 cm | Spindle | Malignant GIST | - | - |
| 4. | 68 | M | Recto-sigmoid | 8.5×7×4.5 cm | Spindle | Malignant GIST | Liver | + |
| 5. | 70 | M | Rectum | 5×5×3 cm | Epithelioid | Benign GIST | - | + |
| 6. | 47 | F | Ileum | 4×3×4 cm | Spindle | Benign GIST | - | + |
| 7. | 45 | F | Stomach | 11×9×8 cm | Mixed | Malignant GIST | - | +++++ |
| 8. | 51 | M | Jejunum | 7.5×5.5×5 cm | Spindle | Benign GIST | - | + |
| 9. | 50 | M | Transverse colon | 8×7.5×6 cm | Spindle | Benign GIST | - | +++++ |
| Extra-gastrointestinal stromal tumours |
| 10. | 55 | M | Mesentery (Stomach) | 5×3×2 cm | Spindle | Benign GIST | - | + |
| 11. | 04 | F | Retro-peritoneum | 15×12×7 cm | Spindle | Malignant GIST | - | +++ |
| 12. | 35 | F | Retro-peritoneum | 21×15×9 cm | Mixed | Benign GIST | - | + |
GIST: Gastrointestinal stromal tumour; M: Male; F: Female; HP: Histopathology
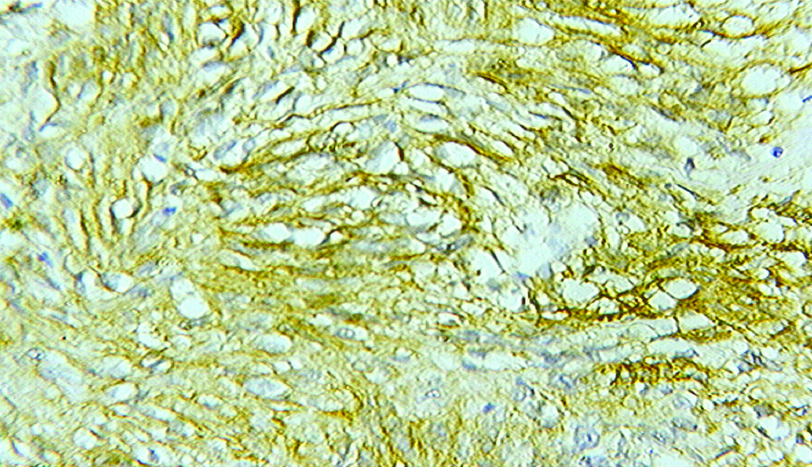
Immunohistochemical study for c-KIT was done in all the cases. c-KIT positivity was found to be positive in all benign GISTs with cytoplasmic staining pattern most commonly found. About 40% (2/5) cases among the malignant GISTs were negative for c-KIT [Table/Fig-5].
IHC X 40. Immunohistochemistry for CD117 (CKIT) shows strong and diffuse cytoplasmic staining.
Discussion
The median age at diagnosis was 66-69 years and the mean age at presentation in this study was 50.6 years, with male to female ratio is 2:1. These observations were similar to the Lakshmi VA et al., and Varshney VK et al., studies [2,4], whereas in other studies it was most common in old age [3].
The clinical presentation is mainly based on the location and size of the lesion, hence, most of the GISTs remain ‘silent’ or often nonspecific until reaching a larger size [10]. The most common presentation of intestinal GIST is GI bleeding which may be acute (melena or haematemesis) or chronic. The commonest presenting symptoms in this series were GI bleeding, abdominal pain, and abdominal mass followed by anorexia and vomiting. This is similar to that observed in other series [2,4]. Larger sized GISTs usually protrude into the lumen from the site of origin or grow between the bowel loops or the abdominal organs. Hence, bleeding may take place either into the abdominal cavity causing acute abdominal pain and severe anaemia and sometimes leading to emergency surgery or into the GIT lumen causing haematemesis, melena and anaemia. Patients with GISTs may also present with nonspecific symptoms such as nausea, vomiting, discomfort, dyspepsia, change of bowel habits, obstructive jaundice, fever and symptoms of anaemia.
Studies of the literature shows stomach (50%-60%) to be the most common site followed by small intestine (20%-30%), large intestine and very rarely in extraintestinal sites. In the present study, the most common site for GISTs was large intestine (33.3%) followed by stomach (25%) which is probably due to differences in population based studies. Hence, extensive studies are required to evaluate this, to support. Most tumours in the present study showed pure spindle cell morphology and few had pure epithelioid and mixed type morphology. These observations were similar to most of the studies like Lakshmi VA et al., Gaopande VL et al., Metastases of GITs commonly develop in the abdominal cavity and liver [2,5], rarely in bones, soft tissues, skin and extremely rarely in lymph nodes and lungs [11] which will be seen mainly in tumours of high and intermediate risk groups. These results were consistent with this study. In this study, two cases had metastasis, one to liver and another with metastasis to iliac crest.
In this study, two cases had metastasis, one to liver and another to iliac crest. In the present study, size of the tumours ranged from 1 cm to more than 40 cm, among which the median size of of GISTs were between 5 cm to 8 cm. and few were >10 cm which were categorised as malignant. Morphologically GISTs show a wide spectrum of morphological patterns from bland spindle cell proliferations to highly cellular epithelioid tumours with significant nuclear pleomorphism. Skeinoid fibres are usually associated with lower-grade lesions and prognosis of GIST is highly associated with mitotic rate, tumour size and anatomical location. Small tumours with < or=2 cm and mitotic activity not exceeding 5/50 HPFs have an excellent prognosis and probably independent of anatomical location, but does not hold good for all locations. Most of the epithelioid GISTs in stomach are benign, where the mitotic counts should be <5/50 HPFs. Hence, mitotic activity and size of tumours were assessed in ordered to assign tumours into risk groups as low, moderate and high risk groups as described by Fletcher CD et al., [12]. A small proportion of tumours apparently lacking mitotic activity do metastasize, however tumours with a mitotic rate >10/50 HPFs usually indicates malignant behaviour [13]. Present study had 57.1% of benign tumours and 42.9% were malignant. The GISTs on imaging studies were usually diagnosed as soft tissue tumours like smooth muscle tumours, inflammatory fibroid polyps, fibromatosis, schwannomas etc., where as in the present study, the differential diagnosis on histopathological examination considered was, leiomyoma, leiomyosarcoma and fibrosarcoma. Hence, the definitive diagnosis is usually made by IHC studies, correlating with histopathological features [14].
Most of the GISTs (95%) show strong and diffuse immunostaining positivity for the KIT protein (CD117 antigen, an epitope of the KIT tyrosine kinase). Majority of the tumours exhibit cytoplasmic staining pattern and in some tumours, a coexisting dot like, or Golgi staining pattern was seen. Very rarely, a membranous staining pattern is observed. Hence c-KIT is a very useful marker in differentiating GIST from other mesenchymal tumours of the GIT, due to its high sensitivity and specificity. Similar observations were seen in the present study with two malignant GISTs which were negative for c-KIT. Another common marker that is not as sensitive or specific for GIST is CD34. This c-KIT is expressed in 70% of GISTs and it was the first immunohistochemical marker that helped to distinguish these tumours from leiomyomas and leiomyosarcomas of the GIT [15]. Smooth muscle actin is found to be positive in nearly 30% to 40% of GISTs. Other markers which show variable and weak immunopositivity are h-caldesmon, S100, desmin, and cytokeratin’s 8 and 18 [2].
Nearly 5% of GISTs are negative for c-KIT by immunohistochemistry. The tumours with epithelioid or mixed historphological features have more affinity for stomach and omentum or peritoneum, hence these lesions tend to harbour either c-KIT wild-type or PDGFRA mutations. The diagnostic accuracy in c-KIT-negative GISTs can be improvised by using several newer markers discovered on gene expression arrays, like DOG1 a calcium-activated chloride channel composed of 8 transmembrane domains is one such marker that is found to be highly expressive in GIST [1,3-5].
Extra-gastrointestinal GISTs share histological and immunohistochemical features of gastrointestinal GIST i.e., staining with c-KIT, a marker of interstitial cells of Cajal which are normally present in the GIT. The reason how these cells reach the omentum, mesentery and retroperitoneum and develop into a tumour is not clear. Most probably, it may be the extensive extramural component that lose contact with gut wall or may arise from the multipotent mesenchymal stem cells [1]. The intracytoplasmic portion of KIT functions as a tyrosine kinase, hence Imatinib mesylate and Sunitinib malate are competitive inhibitors of the ATP binding pocket of KIT and PDGFRA that were approved for first and second line GIST treatment, respectively [16].
Limitation(s)
The limitation of this study includes lack of large panel of molecular markers, small sample size and follow-up. Hence, there is a need for further multicentric studies, large sample size for definitive analysis and to rationalise the treatment of these lesions.
Conclusion(s)
Gastrointestinal Stromal Tumour are considered as the most common mesenchymal tumour of GIT and are frequently present in small intestine and should be considered as a possible cause of GI bleeding, when upper and lower GI endoscopy is found to be normal. Diagnosis of GIST and EGIST depends on both characteristic histopathology and immunohistochemistry. This study emphasises that c-KIT is a reliable and sensitive diagnostic tool for GIST and EGIST, where GISTs show positivity for c-KIT and negativity of the same does not rule out GIST. Hence, it requires demonstration of other markers like PDGFRA and DOG-1 (wild types) along with thorough clinico-radiological and pathological correlation.
UGI: Upper gastrointestina; LGI: Lower gastrointestinal
GIST: Gastrointestinal stromal tumour; M: Male; F: Female; HP: Histopathology