Correlation of Placental Growth Factor with Pre-Eclampsia during Mid Trimester of Pregnancy: A Case Control Study at Liaquat University of Medical and Health Sciences, Pakistan
Khalid Yousuf Memon1, Ikram Din Ujjan2, Nailla Yousuf3, Syed Hasan Ala4, Shabnam Rustamani5, Fozia Shaikh6, Abid Hussain Chang7, Suha Mahrukh8
1 Lecturer, Department of Pathology, Liaquat University of Medical and Health Sciences, Jamshoro, Sindh, Pakistan.
2 Professor, Department of Pathology, Liaquat University of Medical and Health Sciences, Jamshoro, Sindh, Pakistan.
3 Associate Professor, Department of Obstetrics and Gynaecology, Peoples Medical University of Medical and Health Sciences for Womens, Nawab Shah, Pakistan.
4 Assistant Professor, Department of Obstetrics and Gynaecology, Dow University of Health Sciences, Karachi, Pakistan.
5 Lecturer, Department of Pathology, Liaquat University of Medical and Health Sciences, Jamshoro, Sindh, Pakistan.
6 Lecturer, Department of Biochemistry, Liaquat University of Medical and Health Sciences, Jamshoro, Sindh, Pakistan.
7 Associate Professor, Department of Pathology, Liaquat University of Medical and Health Sciences, Jamshoro, Sindh, Pakistan.
8 Final Year Student, Liaquat University of Medical and Health Sciences, Jamshoro, Sindh, Pakistan.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Khalid Yousuf Memon, Lecturer, Department of Pathology, Liaquat University of Medical and Health Sciences, Jamshoro, Sindh, Pakistan.
E-mail: drmsarain@gmail.com
Introduction
Pre-Eclampsia (PE) is the common multisystem disorder which complicates the pregnancies 3-8% and a major cause of morbidity and mortality throughout the world.
Aim
To determine the correlation of placental growth factor with PE in mid trimester of pregnancy at Liaquat University of Medical and Health Sciences Jamshoro.
Materials and Methods
This Case-Control study was performed at Pathology Department with Department of Obstetrics and Gynaecology, Liaquat University of Medical and Health Sciences (LUMHS) Jamshoro/Hyderabad, during 12 months, from 6th February, 2017 to 5th February 2018. Pregnant women with mid trimester were enrolled in the study. Group A included females with PE, while Group B were normotensive females of same gestation. Their blood sample were collected and stored at -80°. Level of placental growth factor was measured on Elecsys system. Data was analysed via Statistical Package for the Social Sciences (SPSS) version 20.
Results: Total 384 PE females were selected and 50 females without hypertension were studied as control. Mean age of patients was 27.46±3.91 years. No significant variance was seen between mean of gestational age of patients and normal pregnant females; p-value 0.346. Mean of placental growth factor was insignificantly decreased 35.21±31.68 pg/mL among patients in contrast to normal women as 47.23±56.13 pg/mL, p=0.081. A negative correlation was found between blood pressure and placental growth factor, r-values -0.004 and -0.001, respectively.
Conclusion
It was concluded that serum placental growth factor was the poor marker for PE, as it showed weak negative correlation with PE.
Hypertension, Marker, Normotensive, Pregnant women
Introduction
PE is the major cause of maternal morbidity and mortality [1]. It is characterised by hypertension, proteinuria and oedema. This further leads to seizures, stroke, intrauterine foetal growth restriction and even may leads to maternal and foetal death [2]. It is further divided as late and early onset PE on the basis of gestational age at presentation, before and after 34 weeks of gestation [3]. Extensive research was done for secondary prevention to control and treat the disease by conservative management that successfully reduced maternal morbidity and mortality on the cost of early delivery, hence increased neonatal prematurity and related perinatal morbidity and mortality [1,4,5]. Nevertheless, primary prevention to reduce its prevalence by understanding the pathology is unclear [6]. It has been asserted that during embryogenic period, dysfunction related to placental vascularisation and angiogenesis contribute significantly to the progression of this disease. Vascular Endothelial Growth Factor family (VEGF) that includes Placental Growth Factors (PGF) is key factor which was claimed to be involved in PE [7]. PGF, a glycoprotein part of VEGF family, is synthesised by placenta, which stimulates propagation, migration and triggering of endothelial cells. It has been postulated that impaired placentation in pregnancy leads to reduced maternal serum PGF. This reduction is evident from 11 weeks onwards [8]. It has been argued that these biochemical markers are released in response to placenta ischaemia [9]. sFlt-1, a tyrosine kinase protein and a variant of VEGF receptor 1 (R1), interferes with growth of blood vessel and it neutralises the action of PGF through interaction of pro-angiogenic factors that act as endothelial receptors and impedes endothelial function that propagates to PE [9-11]. Raised level of angiogenic biochemical markers distinguish pre-eclamptics from normotensive pregnant patients [11,12]. In the course of recent years, PGF has been exhibited to be reduced among pregnant females with PE [13,14]. Reduced plasma concentrations of PGF are detectable at the time of PE [15]. Both factors have been seen to be correlated with raised risk of PE [16,17]. However, there is limited number of studies in this regard and no local study was found, despite of explicit search. Therefore, the purpose of present study was to assess the correlation of PGFs with PE in present setup, which not only prevents foeto-maternal mortality and morbidity, but also saves cost and resources spent for the admission and treatment of this catastrophic disease.
Materials and Methods
This Case-Control study was carried out at Pathology Department with Department of Obstetrics and Gynaecology, Liaquat University of Medical and Health Sciences (LUMHS), Jamshoro/Hyderabad, during 12 months from 6th February, 2017 to 5th February, 2018. Ethical approval was taken (No. LUMHS/REC/272) before data collection. Sample size was calculated by taking the 30% prevalence of pre-ecmplsia [15], with 95% confidence interval. Thus, the sample size calculated was 384 pregnant women, out of them 50 normal women were selected as controls. All singleton pregnant women with mid trimester were enrolled in the study. The females with medical conditions like systemic lupus erythematosus, renal disease, diabetes mellitus, Antiphospholipid antibody syndrome, or any known autoimmune disease and users of chronic corticosteroid drug were excluded from this study. Pre-eclampsia was determined by systolic blood pressure (BP) ≥140 mmHg or a diastolic BP ≥90 mmHg on two successive measurements. Maternal plasma specimens were isolated via 2,500 rpm centrifugation for 10 minutes. Aliquots of maternal plasma were stored at -80°. Levels for PGF were measured on fully automated Elecsys system. This assay is a sandwich immunoassays grounded on the electrochemiluminescence technology. The cut-off PGF values was set at 15.6-pg/mL as mentioned by manufacturer.
Statistical Analysis
All the information was recorded in self-made proforma. Analysis of data was done by a Statistical software Statistical Package for the Social Sciences (SPSS) version 20. Student t-test was applied and a p-value ≤0.05 was considered as significant.
Results
In this study, 384 females with PE and 50 normotensive pregnant females as control were enrolled. Mean age of patients were 27.46±3.91 years and control’s was 28.0±4.0 years, p-value 0.362. Mean gestational age of pre-eclamptic women was 25.08±2.34 weeks and controls were 25.42±2.61 weeks. Mean of the blood pressure was markedly higher as compared to normal women with p-value 0.0001 [Table/Fig-1].
Distribution of patients according to demographic characteristics n=434.
| Demographics | Pre-eclampsia n=384 | Control n=50 | p-value |
|---|
| Age | 27.46±3.91 years | 28.0±4.0 years | 0.362 |
| Gestational age | 25.08±2.34 weeks | 25.42±2.61 weeks | 0.346 |
| Systolic BP* | 147.43±10.83 mmHg | 111.90±6.91 mHg | 0.001 |
| Diastolic BP* | 103.15±6.39 mmHg | 72.10±7.36 mmHg | 0.001 |
*BP: Blood pressure; p-value ≤0.05 was considered as significant.
Urinary proteins among pre-eclamptics and controls were statistically significant. Mean of PGF was found to be lower in patients with PE while higher in normotensives; however statistically nonsignificant, p-value 0.081 [Table/Fig-2].
Distribution according to mean of urinary protein and Placental Growth Factor (PGF) n=434.
| Parameters | Blood pressure | p-value |
|---|
| Pre-eclampsia n=384 | Control n=50 |
| PGF | Mean | 35.21±31.68 pg/mL | 47.23±25.13 pg/mL | 0.081 |
| Urinary protein | Mean | 1.54±0.72 mg/L | 1.14±0.35 mg/L | 0.001* |
*p-value ≤0.05 was considered as significant
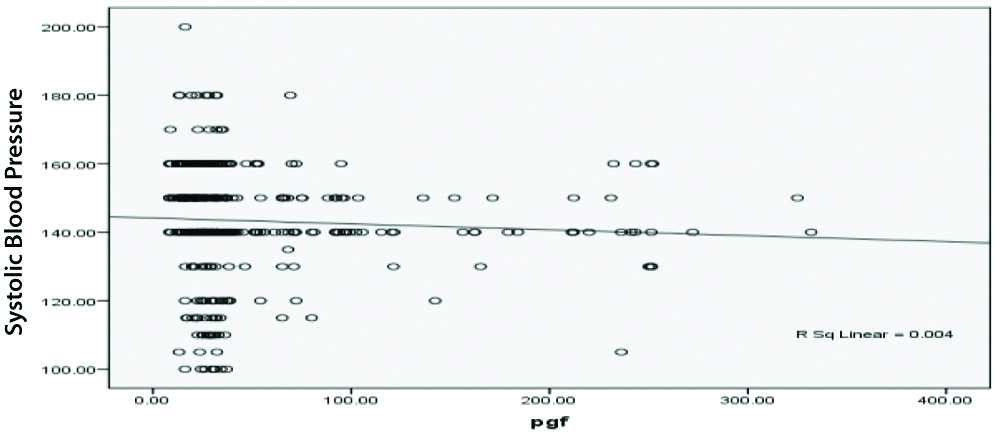
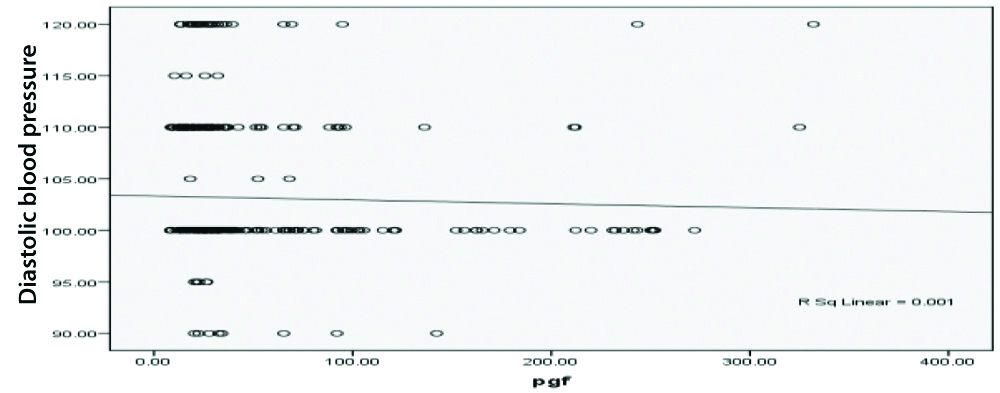
There was a weak negative correlation between Systolic BP and placental growth hormone, r-value-0.004 [Table/Fig-3]. There was also a weak negative correlation amid diastolic BP and placental growth hormone, r-value-0.001 [Table/Fig-4].
Pearson correlation between Placental Growth Factor (PGF) and systolic blood pressure.
Pearson correlation between PGF and diastolic blood pressure.
Discussion
Placental Growth Factors is a predictive marker in development of PE, but limited data is available [18]. In this study, statistically no significant differences were found in basic characteristics among pregnant females who developed PE compared to normotensives. The mean age of patients was 27.46±3.91 years, which was almost similar to controls, p-value 0.362. A comparable study of Shibata E et al., reported that mean age of women with PE was 26.5±6.0 years and mean age of normal women was 26.2±7.1 years, which was statistically insignificant [19]. In this study, there was no significant variance between mean gestational age among both groups, p-value 0.346. Inconsistently, Hanita O et al., observed that in pre-eclamptics females, the gestational age at delivery was significantly lesser than those who didn’t develop the condition (p<0.0005) [18]. This difference can possibly be because in present study, patients were with 2nd trimester, which is the lower gestational age as compared to Hanita O et al., [18].
In this study, mean placental growth factor was insignificantly decreased 35.21±31.68 pg/mL in patients, while in normotensives it was 47.23±25.13, p-value 0.081. Similar findings were found in a study of Gurnadi JI et al., [20]. This was further endorsed by Schmidt M et al., who reported that PGF levels from 15 to 18 weeks of gestational age were markedly lower than normal women [21]. Using a PGF level of 41.84 pg/mL as a cut-off, this test has a sensitivity of 0.87 and a specificity of 0.83 [21]. Sibiude J et al., reported that Mean PGF values (following log transformation) were significantly lower in cases those later developed PE than the subjects who didn’t develop PE [22]. Lower values of PGF were as well significantly correlated with adverse outcome [22].
The present study exhibited PGF as a poor indicator of PE in mid trimester of pregnancy because there was weak negative correlation between systolic and diastolic BP and PGF (r-values-0.004 and -0.001, respectively) with 70% sensitivity and 60% specificity. Similarly, in the study of Elsheikh WA et al., ROC curve analysis for the probability of PGF to predict PE revealed that PGF can predict PE with sensitivity of 74%, specificity 50% [23]. Kleinrouweler CE et al., [24] conducted a systemic review and meta-analysis to examine the capacity of circulating PGF and other angiogenic factors to predict PE and reported that PGF exhibited modest however significantly different concentrations prior to 30 weeks of gestation among females who developed PE, nonetheless, the test accuracy (32% sensitivity) was worse for precise prediction of PE within clinical practice [24]. Conde-Agudelo ARR and Roberts JM, postulated that females have moderate to low risk to develop PE, the prognostic accuracy of angiogenic factors including PGF was high to moderate when measured in the course of the 2nd trimester (sensitivities ranging from 100% to 17%, specificities from 97% to 51%) [25]. On the other hand, many authors showed that low level of maternal PGF precede the clinical presentation of PE, provide a good indicator of SGA and severe PE and predict women who will develop early-onset PE [26,27]. However, McElrath TF et al., reported sensitivity 47% and specificity 62%, poor predictive value of PGF for prediction of PE [28]. In another study of Sung KU et al., observed that PGF is the useful marker during 1st trimester for hypertensive disorders in pregnancy [29]. However, PGF during 1st trimester may be more useful, though further large-scale studies of both, mid and first trimester are needed for further evaluation of the usefulness of this marker.
Limitation(s)
This study was single center study and only mid trimester women were included. Another limitation is lack of funding sources due to which control cases ratio could not maintained.
Conclusion(s)
It was concluded that serum plasma growth factor is non-significant for PE, as it showed weak negative correlation with pre-eclampsia. Large prospective multicenter studies are recommended to ascertain the association of these biochemical markers with pre-eclampsia before employment in clinical practice.
*BP: Blood pressure; p-value ≤0.05 was considered as significant.
*p-value ≤0.05 was considered as significant
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 09, 2020
Manual Googling: Oct 17, 2020
iThenticate Software: Oct 29, 2020 (12%)
[1]. Fondjo LA, Boamah VE, Fierti A, Gyesi D, Owiredu EW, Knowledge of pre-eclampsia and its associated factors among pregnant women: A possible link to reduce related adverse outcomesBMC Preg Child B 2019 19(1):45610.1186/s12884-019-2623-x31791264 [Google Scholar] [CrossRef] [PubMed]
[2]. Bujold E RS, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest JC, Prevention of pre-eclampsia and intrauterine growth restriction with aspirin started in early pregnancy: A meta-analysisObstet Gynecol 2010 116(2):402-14.10.1097/AOG.0b013e3181e9322a20664402 [Google Scholar] [CrossRef] [PubMed]
[3]. Mutter WP, Karumanchi SA, Molecular mechanisms of pre-eclampsiaMicrovasc Res 2008 75(1):01-08.10.1016/j.mvr.2007.04.00917553534 [Google Scholar] [CrossRef] [PubMed]
[4]. Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E, Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: A meta-analysisUltrasound Obstet Gynecol 2013 41(5):491-99.10.1002/uog.1242123362106 [Google Scholar] [CrossRef] [PubMed]
[5]. Langenveld J, Broekhuijsen K, van Baaren GJ, van Pampus MG, van Kaam AH, Groen H, Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia between 34 and 37 weeks’ gestation (HYPITAT-II): A multicentre, open-label randomised controlled trialBMC Pregnancy Childb 2011 11(1):01-07.10.1186/1471-2393-11-5021736705 [Google Scholar] [CrossRef] [PubMed]
[6]. Amin O, Tasnim N, Naeem S, Prevention of pre-eclampsia with low dose aspirin in primigravidaJ Womens Health 2020 9(1):28-32.10.15406/mojwh.2020.09.00264 [Google Scholar] [CrossRef]
[7]. Hertig A LP, New markers in pre-eclampsiaClinica Chimica Acta 2010 411:1591-95.10.1016/j.cca.2010.07.02020659441 [Google Scholar] [CrossRef] [PubMed]
[8]. Llurba E, Syngelaki A, Sánchez O, Carreras E, Cabero L, Nicolaides KH, Maternal serum placental growth factor at 11-13 weeks’ gestation and fetal cardiac defectsUltrasound Obstet Gynecol 2013 42(2):169-74.10.1002/uog.1234623151971 [Google Scholar] [CrossRef] [PubMed]
[9]. Friedman SA dGC, Taylor RN, Golditch BD, Roberts JM, Plasma cellular fibronectin as a measure of endothelial involvement in pre-eclampsia and intrauterine growth retardationAm J Obstet Gynecol 1994 170(3):838-41.10.1016/S0002-9378(94)70295-0 [Google Scholar] [CrossRef]
[10]. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in pre-eclampsiaJ Clin Invest 2003 111(5):649-58.10.1172/JCI1718912618519 [Google Scholar] [CrossRef] [PubMed]
[11]. De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D’anna R, Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsiaActa Obstet Gynecol Scand 2008 87(8):837-42.10.1080/0001634080225375918607829 [Google Scholar] [CrossRef] [PubMed]
[12]. Yelumalai S KSS, Qvist R, Muniandy S, Angiogenic factors in the pathogenesis and pathophysiology of Pre-eclampsia: A mini reviewBiomed Res 2010 21(3):246-51. [Google Scholar]
[13]. Leaños-Miranda A, Campos-Galicia I, Isordia-Salas I, Rivera-Leaños R, Romero-Arauz JF, Ayala-Méndez JA, Changes in circulating concentrations of soluble fms-like tyrosine kinase-1 and placental growth factor measured by automated electrochemiluminescence immunoassays methods are predictors of pre-eclampsiaInt. J. Hypertens 2012 30(11):2173-81.10.1097/HJH.0b013e328357c0c922902831 [Google Scholar] [CrossRef] [PubMed]
[14]. Hassan MF, Rund NM, Salama AH, An elevated maternal plasma soluble fms-like tyrosine kinase-1 to placental growth factor ratio at midtrimester is a useful predictor for pre-eclampsiaInt J Gynaecol Obstet 2013 2013:20234610.1155/2013/20234624367379 [Google Scholar] [CrossRef] [PubMed]
[15]. Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for pre-eclampsiaJ Clin Endocrinol Metab 2004 89(2):770-75.10.1210/jc.2003-03124414764795 [Google Scholar] [CrossRef] [PubMed]
[16]. Rizos D, Eleftheriades M, Karampas G, Rizou M, Haliassos A, Hassiakos D, Placental growth factor and soluble fms-like tyrosine kinase-1 are useful markers for the prediction of pre-eclampsia but not for small for gestational age neonates: A longitudinal studyEur J Obstet Gynecol Reprod Biol 2013 171(2):225-30.10.1016/j.ejogrb.2013.08.04024035323 [Google Scholar] [CrossRef] [PubMed]
[17]. Ding G, Liping L, Moli D, Wuliyeti A, Shaohe Z, Huijuan W, A study of the association between the sFlt-1/PIGF ratio and pre-eclampsia in Xinjiang Uygur Autonomous Region of ChinaArtif Cell Nanomed B 2018 46:281-86.10.1080/21691401.2018.149148030831776 [Google Scholar] [CrossRef] [PubMed]
[18]. Hanita O, Alia NN, Zaleha AM, Azlin MN, Serum soluble FMS-like tyrosine kinase 1 and placental growth factor concentration as predictors of pre-eclampsia in high risk pregnant womenMalays J Pathol 2014 36(1):19 [Google Scholar]
[19]. Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Soluble fms-like tyrosine kinase 1 is increased in pre-eclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: Relationship to circulating placental growth factorJ Clin Endocrinol Metab 2005 90(8):4895-903.10.1210/jc.2004-195515886253 [Google Scholar] [CrossRef] [PubMed]
[20]. Gurnadi JI, Mose J, Handono B, Satari MH, Anwar AD, Fauziah PN, Difference of concentration of placental soluble fms-like tyrosine kinase-1 (sFlt-1), placental growth factor (PlGF), and sFlt-1/PlGF ratio in severe pre-eclampsia and normal pregnancyBMC Res Notes 2015 8(1):53410.1186/s13104-015-1506-026434493 [Google Scholar] [CrossRef] [PubMed]
[21]. Schmidt M, Dogan C, Birdir C, Kuhn U, Gellhaus A, Kimmig R, Placental growth factor: A predictive marker for pre-eclampsia?Gynäkol Geburtshilfliche Rundsch 2009 49(2):94-99.10.1159/00019790819346754 [Google Scholar] [CrossRef] [PubMed]
[22]. Sibiude J, Guibourdenche J, Dionne MD, Le Ray C, Anselem O, Serreau R, Placental growth factor for the prediction of adverse outcomes in patients with suspected pre-eclampsia or intrauterine growth restrictionPloS one 2012 7(11):e5020810.1371/journal.pone.005020823209675 [Google Scholar] [CrossRef] [PubMed]
[23]. Abdelgalil Elsheikh W, Behery M, Farag E, Hassan F, Abdelaziz F, Alkholy M, The role of second trimester uterine artery doppler ultrasound, inhibin-a and placental growth factor in prediction of pre-eclampsiaZagazig Vet J 2016 22(4):01-01.10.21608/zumj.2016.4651 [Google Scholar] [CrossRef]
[24]. Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, Bossuyt PM, van der Post JA, von Dadelszen P, Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: A systematic review and meta-analysisBJOG: Int J Gynaecol Obstet 2012 119(7):778-87.10.1111/j.1471-0528.2012.03311.x22433027 [Google Scholar] [CrossRef] [PubMed]
[25]. Conde-Agudelo A RR, Roberts JM, Tests to predict pre-eclampsiaIn Chesley’s hypertensive disorders in pregnancy 2015 1Academic Press:221-51.10.1016/B978-0-12-407866-6.00011-0 [Google Scholar] [CrossRef]
[26]. Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop pre-eclampsia and deliver a small for gestational age neonateJ Matern Fetal Neonatal Med 2008 21(1):09-23.10.1080/1476705070183048018175241 [Google Scholar] [CrossRef] [PubMed]
[27]. Villa PM, Hämäläinen E, Mäki A, Räikkönen K, Pesonen AK, Taipale P, Vasoactive agents for the prediction of early-and late-onset pre-eclampsia in a high-risk cohortBMC Pregnancy and Child B 2013 13(1):11010.1186/1471-2393-13-11023663420 [Google Scholar] [CrossRef] [PubMed]
[28]. McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, Longitudinal evaluation of predictive value for pre-eclampsia of circulating angiogenic factors through pregnancyAmerican journal of obstetrics and gynecology 2012 207(5):407-e1.10.1016/j.ajog.2012.08.01022981320 [Google Scholar] [CrossRef] [PubMed]
[29]. Sung KU, Roh JA, Eoh KJ, Kim EH, Maternal serum placental growth factor and pregnancy-associated plasma protein A measured in the first trimester as parameters of subsequent pre-eclampsia and small-for-gestational-age infants: A prospective observational studyObstet Gynecol Sci 2017 60(2):154-62.10.5468/ogs.2017.60.2.15428344956 [Google Scholar] [CrossRef] [PubMed]