This study compares the histological findings in placentas from LBW and normal birth weight delivery and finds the maternal risk factors associated with LBW baby thus, providing scope for management of further pregnancies.
Materials and Methods
This case-control study was conducted in SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu, India between August 2017 to August 2019 after getting approval from Institutional Scientific and Ethical Committee (Ethical clearance number: 1377/IEC/2018).
Inclusion criteria: Placentas from deliveries with live baby weighing less than 2.5 kg was included in group 1(low birth weight) and from deliveries with live baby weighing more than 2.5 kg was included in group 2 (normal birth weight).
Exclusion criteria: Placental samples from preterm delivery, still birth and intrauterine death cases were excluded from the study.
After excluding the cases according to exclusion criteria, consecutive 70 placentas from LBW delivery and 70 placentas from normal birth weight delivery were collected. Sample size was calculated using the formula n=4 pq/L2, p-prevalence, q-100-p, L-allowable error/precision.
Placenta was placed on a flat surface with maternal surface facing upwards and parallel sections were made with a sharp knife at an interval of 5 cm. Tissue samples of 2 cm thickness were taken such that it included both maternal and foetal surface after fixing the specimen in 10% formalin for 24 hours. Three micron sections were taken after tissue processing. The Haematoxylin and Eosin (H&E) stained slides were used for studying histological features like syncytial knots, PVFD, chorioamnionitis, stromal fibrosis and calcification. Basement membrane thickening was studied with Periodic Acid Schiff (PAS) stained slides.
Statistical Analysis
Statistical analysis was done with SPSS version 17. Independent sample Student’s t-test and Chi-square test were used for comparison between two groups.
Results
One hundred and forty placenta specimens were collected during the study period satisfying the inclusion and exclusion criteria. Most common maternal age group in this study was 21-25 years. 37 cases (52.9%) from group 1 and 29 cases (41.4%) from group 2 belonged to this age group [Table/Fig-1].
Maternal age comparison between group 1 (LBW) and group 2 (NBW).
| Maternal age (years) | Count and percentage | Group 1 (LBW) | Group 1 (NBW) | Total |
|---|
| ≤20 | Count | 7 | 8 | 15 |
| % within group | 10.0% | 11.4% | 10.7% |
| 21-25 | Count | 37 | 29 | 66 |
| % within group | 52.9% | 41.4% | 47.1% |
| 26-30 | Count | 23 | 26 | 49 |
| % within group | 32.9% | 37.1% | 35.0% |
| >30 | Count | 3 | 7 | 10 |
| % within group | 4.3% | 10.0% | 7.1% |
| Total | Count | 70 | 70 | 140 |
| % within group | 100.0% | 100.0% | 100.0% |
Pearson Chi-square test p-value 0.420 (non-significant); LBW: Low birth weight; NBW: Normal birth weight.
The mean birth weight of LBW babies was 2.06 kg. The mean placental weight in deliveries with LBW baby was 353.04 gm which was significantly lower than mean placental weight of normal birth weight babies [Table/Fig-2].
Mean values of birth weight and placental weight in group 1 and group 2.
| Group | Mean | SD | p-value |
|---|
| Birth weight (Kg) | Group 1 (LBW) | 2.067 | 0.2821 | <0.0001 |
| Group 2 (NBW) | 3.268 | 0.4720 |
| Placental weight (gm) | Group 1 (LBW) | 353.04 | 81.207 | <0.0001 |
| Group 2 (NBW) | 530.39 | 35.062 |
p-value<0.05 was considered statistically significant; LBW: Low birth weight; NBW: Normal birth weight.
The commonest risk factor associated with LBW baby in this study was anaemia. In this study, 25 mothers (35.7%) belonging to group 1 had anaemia, followed by Pregnancy Induced Hypertension (PIH) which was seen in 12 mothers (17.1%), whereas in group 2-11 cases (15.7%) were associated with anaemia and 4 cases (5.7%) had PIH [Table/Fig-3].
Comparison of maternal high risk factors.
| High risk factors | Count and percentage | Group 1 (LBW) | Group 1 (NBW) | Total |
|---|
| Anaemia | Count | 25 | 11 | 36 |
| % within group | 35.7% | 15.7% | 25.7% |
| GDM | Count | 5 | 1 | 6 |
| % within group | 7.1% | 1.4% | 4.3% |
| Oligohydramnios | Count | 2 | 1 | 3 |
| % within group | 2.9% | 1.4% | 2.1% |
| PIH | Count | 12 | 4 | 16 |
| % within group | 17.1% | 5.7% | 11.4% |
| Nil | Count | 26 | 53 | 78 |
| % within group | 37.1% | 75.7% | 55.7% |
| Total | Count | 70 | 70 | 140 |
| % within group | 100.0% | 100.0% | 100.0% |
GDM: Gestational diabetes mellitus; PIH: Pregnancy induced hypertension; Pearson Chi-square test p-value is <0.0001 was considered statistically significant
Histological findings were compared between both the groups and were found to be frequent in group 1 that is associated with LBW. The most common histological change in this study was villous stromal fibrosis 58 cases (82.9%). Second commonest finding was syncytial knots 57 cases (81.5%) [Table/Fig-4].
Comparison of histological findings between group 1 and group 2.
| Histological findings | Group 1 (LBW) n=70 | Group 2 (NBW) n=70 | Odds ratio | p-value |
|---|
| Chorioamnionitis | 45 (64.3%) | 26 (37.1%) | 3.02 | 0.0015 |
| Syncytial knots | 57 (81.4%) | 15 (21.4) | 16.07 | <0.0001 |
| Calcification | 56 (80%) | 12 (17.1%) | 19.33 | <0.0001 |
| Perivillous fibrin deposition | 55 (78.6%) | 13 (18.5%) | 16.07 | <0.0001 |
| Stromal fibrosis | 58 (82.9%) | 13 (18.5%) | 21.19 | <0.0001 |
| Necrosis | 50 (71.4%) | 28 (40%) | 3.75 | 0.0002 |
| Basement membrane thickening | 49 (70%) | 10 (14.2%) | 14 | <0.0001 |
LBW: Low birth weight; NBW: Normal birth weight. Statistical analysis: Chi-square test; p-value <0.05 was considered statisticallysignificant.
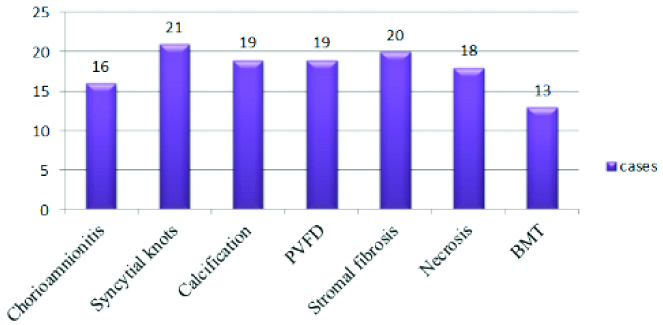
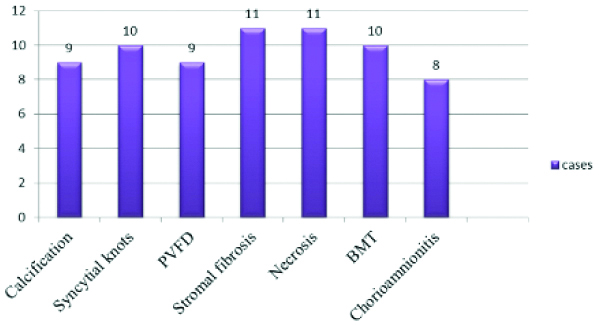
The most common histological finding in the 25 cases which had anaemia as maternal risk factor was excessive syncytial knots which was seen in 21 cases (84%), followed by stromal fibrosis that was seen in 20 cases (80%) [Table/Fig-5]. The second most common maternal high risk factor in present study was PIH. Out of 12 cases of PIH the most common microscopic findings were necrosis and stromal fibrosis. Eleven cases (91.6%) showed stromal fibrosis and necrosis, 10 cases (83.3%) showed syncytial knots and basement membrane thickening [Table/Fig-6].
Histological findings in anaemia complicating pregnancy.
PVFD: Perivillous fibrin deposition; BMT: Basement membrane thickening
Histological findings in PIH complicating pregnancy.
PVFD: Perivillous fibrin deposition; BMT: Basement membrane thickening
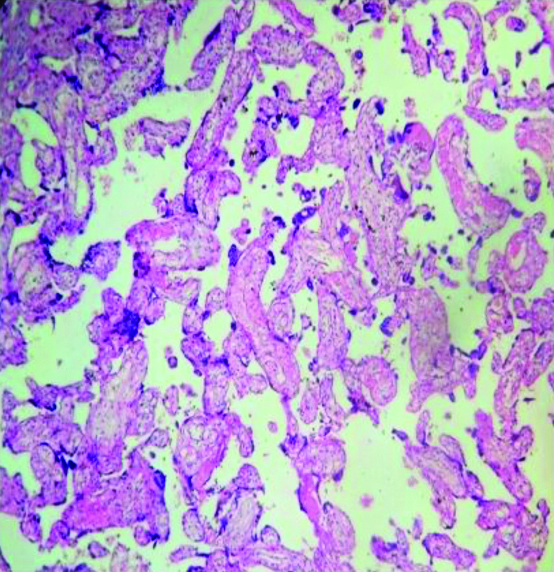
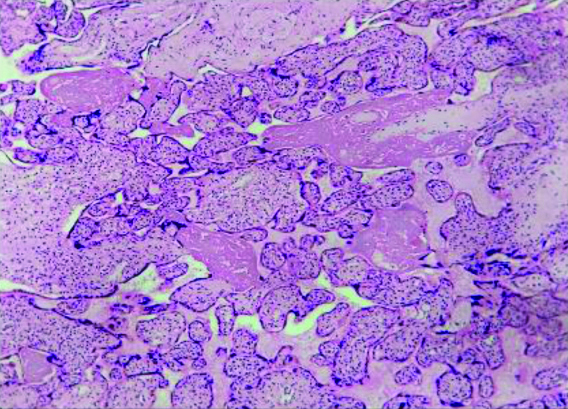
Maternal risk factors associated with LBW baby was analysed in this study. Out of the 70 mothers from the group 1, 44 (62.8%) of them had an associated maternal risk factor. Out of 70 mothers from the group 2, 17 (24.2%) had associated maternal risk factor [Table/Fig-3]. The various histopathological slides showing syncytial knots, stromal fibrosis, necrosis, basement membrane thickening is shown in [Table/Fig-7,8,9 and 10].
Increased syncytial knots in chorionic villi (HPE- H&E x100). Image from Low Birth Weight (LBW) group placenta.
Placental parenchyma showing villous stromal fibrosis (HPE- H&E x100). Image from Low Birth Weight (LBW) group placenta.
Placental parenchyma with necrosis (H&E-x100). Image from Low Birth Weight (LBW) group placenta.
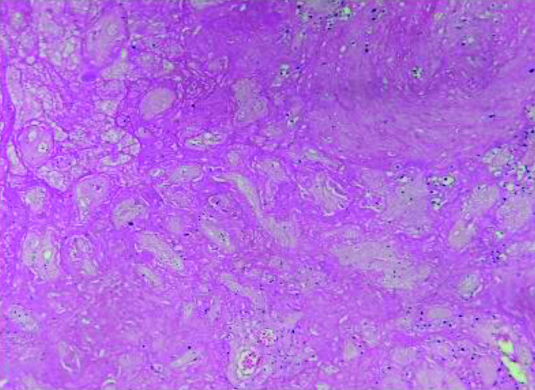
Section showing basement membrane thickening (HPE- Periodic Acid Schiff x100). Image from Low Birth Weight (LBW) group placenta.
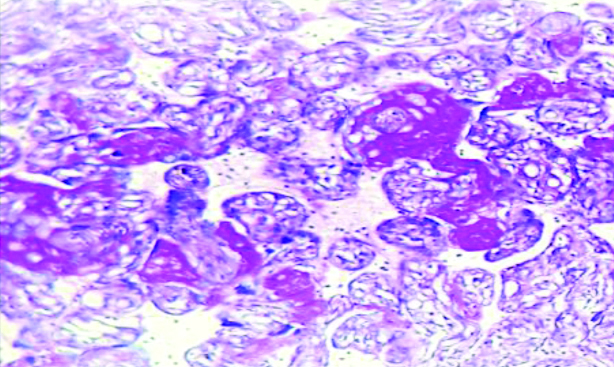
Discussion
LBW has severe consequences in growth and development of the baby. It is the second commonest cause of perinatal death. The impact of LBW depends on the specific cause for foetal growth restriction [7]. In this study the maternal risk factors and histological changes associated with LBW delivery were studied.
In this study, maternal age was divided into four categories as <20 years, 21-25 years, 26-30 years and >30 years. The commonest maternal age group delivering LBW baby in this study was 21-25 years which is similar to the study done by Marvin-Dowle K et al., Eiríksdóttir VH et al., [11,12]. Few studies showed that advancing maternal age (>35 years) has higher risk of delivering LBW baby [13]. Maternal age by itself is not an independent risk factor for LBW. Socio-economic status is closely associated with maternal age making it one of the risk factors for LBW [12]. A <25 years age group is commonly affected due to the changes in socio-economic status according to the study done by Eiríksdóttir VH et al., [12]. The mean placental weight among the LBW cases in present study was 353.04 gm and that of normal birth weight baby was 530.39 gm. The normal weight of placenta is 500 gm [14]. The placental weight and birth weight has a ratio of 1:6 [14]. In this study, the placental weight was found to be reduced in LBW baby which was similar to the study done by Susmita S et al., [15]. In the study done by Susmita S et al., the mean placental weight for LBW baby was 404 gm which was slightly higher than present study and that of normal birth weight baby was 547 gm which was quite similar to present study. Statistically significant difference was observed in placental weight between group 1 and group 2.
Anaemia was the most common risk factor for LBW delivery in present study which was similar to the study done by Rahmati S et al., [16]. Anaemia by itself is a risk factor for LBW baby. According to Rahmati S et al., chance of LBW increases when maternal anaemia occurs in first trimester of pregnancy [16]. The second most common risk factor associated with LBW baby in this study was PIH. This was similar to the study done by Xiao R et al., [17]. According to Xiao R et al., pre-eclampsia has 30.7 times increased risk for Very Low Birth Weight (VLBW) baby (<1500 gm) and 3.8 times increased risk for LBW baby [17].
The histological changes were found to be significantly associated with LBW delivery. Chorioamnionitis was found in 45 (64.3%) cases in group 1 in this study. Chorioamnionitis is due to intra uterine infection (ascending infection) [10]. In this study chorioamnionitis had significant association with LBW baby similar to the study done by Jain A and Dhamnani G [18]. Syncytial knots are because of reduction in uteroplacental blood flow [19]. According to literature knotting of syncytium is significant if it is found in >30% of villi. In this study out of 70 cases, 57 (81.4%) cases showed >30% villi showing syncytial knots [Table/Fig-7]. The findings were similar to the study done by Chandra P et al., where 60-80% of placentas from LBW deliveries showed syncytial knots [19].
In this study, 55 cases (78.6%) of group 1 showed the presence of PVFD. This was found to be similar to the study done by Jain A and Dhamnani G [18]. The reduced blood flow because of PVFD causes placental infarction. PVFD was seen in 62.5% of cases and it was the most common finding in preterm babies according to Jain A and Dhamnani G [18]. In term placenta stromal fibrosis involving <3% is normal [20]. In this study 58 cases (82.9%) showed stromal fibrosis >3% which was similar to the study done by Jadhav CR et al., [Table/Fig-8] [21]. Up to 3% of the placental villi show fibrinoid necrosis in normal placenta [22]. According to the study done by Jadhav CR et al., fibrinoid necrosis was seen in all the cases of LBW placenta [21]. In present study, 50 cases (71.4%) of LBW placenta showed necrosis [Table/Fig-9]. In present study 49 cases (70%) showed thickening of basement membrane which was similar to the study done by Chaithra R Jadhav CR [Table/Fig-10] [21]. According to the literature basement membrane thickening is associated with IUGR, diabetes mellitus and pre-eclampsia [10].
Out of the 25 LBW cases associated with maternal anaemia in present study the most common histological finding was excessive syncytial knotting which was found in 21 (84%) cases followed by stromal fibrosis found in 20 (80%) cases. This was similar to the study done by Adil S and Nausheen Rumana AK [23]. Out of the 12 cases with PIH, 11 cases (91.65%) showed stromal fibrosis and necrosis which was similar to the study done by Dhabhai P et al., [24].
Limitation(s)
Follow-up of the baby for complications could not be done because of irregular post-natal visits. The type and degree of maternal anaemia more prone to deliver low birth baby could not be ascertained.
Conclusion(s)
Growth and development of the foetus depends on the development, formation and maturation of placenta. Placenta plays an important role in foetal weight gain. This study highlighted the commonest histological changes in placenta associated with LBW baby. It also correlated LBW with associated maternal risk factors. The histopathological examination of LBW placenta showed chorioamnionitis, syncytial knots, calcification, PVFD, villous stromal fibrosis, necrosis and basement membrane thickening. The commonest histological finding in this study was villous stromal fibrosis followed by excessive syncytial knots. Histological changes associated with risk factors were studied and it was found that excessive syncytial knots dominate in anaemia group and stromal fibrosis and necrosis dominates in PIH group. This study showed the presence of significant histological changes in placenta of LBW baby and provides scope for further studies in preventing morbidity in LBW delivery.
Pearson Chi-square test p-value 0.420 (non-significant); LBW: Low birth weight; NBW: Normal birth weight.
p-value<0.05 was considered statistically significant; LBW: Low birth weight; NBW: Normal birth weight.
GDM: Gestational diabetes mellitus; PIH: Pregnancy induced hypertension; Pearson Chi-square test p-value is <0.0001 was considered statistically significant
LBW: Low birth weight; NBW: Normal birth weight. Statistical analysis: Chi-square test; p-value <0.05 was considered statisticallysignificant.