Globally, CRC is the third most common cancer with 1.8 million new cases per year and a prevalence rate of 47 million per five years [1,2]. In the year 2012, an estimated death due to CRC was approximately 7 lacs. According to Globocon 2018 there were total of 27 thousand new cases of CRC in India [1]. CRC is common in North America, Australia, and Europe when compared to other parts of the globe. A higher burden of CRC is thought to be due to westernised habits [3,4]. In contrast, registries in Asia, Africa, and South America have the lower rates of CRC’s [3]. The typical Indian diet is lower in calories consisting of more fruits and green leafy vegetables. This along with increased physical activity results in lower obesity rates [5,6]. Unfortunately, even inspite of low incidence rates in India, CRC is associated with a low five year survival rate.
For deciding treatment and prognosis, clinicopathological staging of CRC is carried out using the American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) Tumour-Node-Metastasis (TNM) staging system [7,8]. However, a good number of CRC’s demonstrate local or distant recurrence in spite of being categorised as low risk by the TNM system. This failure of TNM staging to serve as a reliable prognostic method for patients with intermediate-stage tumours may be overcome by other factors like morphology, molecular methods or treatment that can stratify patients more precisely into different categories of risk.
Thus, the search for additional methods to assess the prognosis of patients with CRC had been a major research focus [7,9]. The routine use of histological prognostic factors like tumour depth, tumour differentiation, Lympho-Vascular Invasion (LVI), Perineural Invasion (PNI) and tumour budding to demonstrate the risk of nodal dissemination in CRC is limited by their high inter-observer variability and low standards. More recently, a novel histological grading system has been highlighted as a histopathological prognostic indicator of CRC. This system considers PDC as clusters composed of ≥5 cancer cells present at the invasive front of the tumour lacking full gland formation, and it may predict the metastatic potential of CRC. PDC assessment in comparison to above mentioned histological predictors is considered more promising [10].
Hence, the present study was done to compare PDC based grading with conventional WHO grading system in resected specimens of CRC’s and to evaluate its relationship with known prognostic histopathological parameters such as TNM staging, LVI, PNI, Desmoplastic Reaction (DR) and Crohn’s Like Lymphoid Reaction (CLR).
Materials and Methods
It was a descriptive study done in the Department of Pathology, JSS Medical College and Hospital, Mysuru, Karnataka, India, for duration between September 2015 to March 2019.
Sample size estimation was done using formula- S=4 PQ/d2 where, P=Prevalence (from previous studies), Q=100-P, d=allowable error (5-20% of P). Sixty histopathologically diagnosed CRC’s including heterogenous CRC’s were included in the study and cases with preoperative neoadjuvant chemotherapy were excluded.
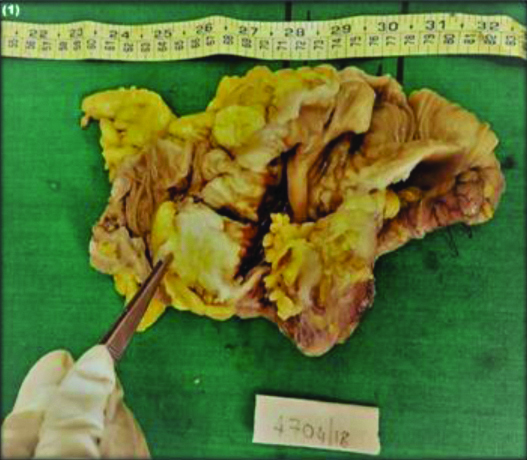
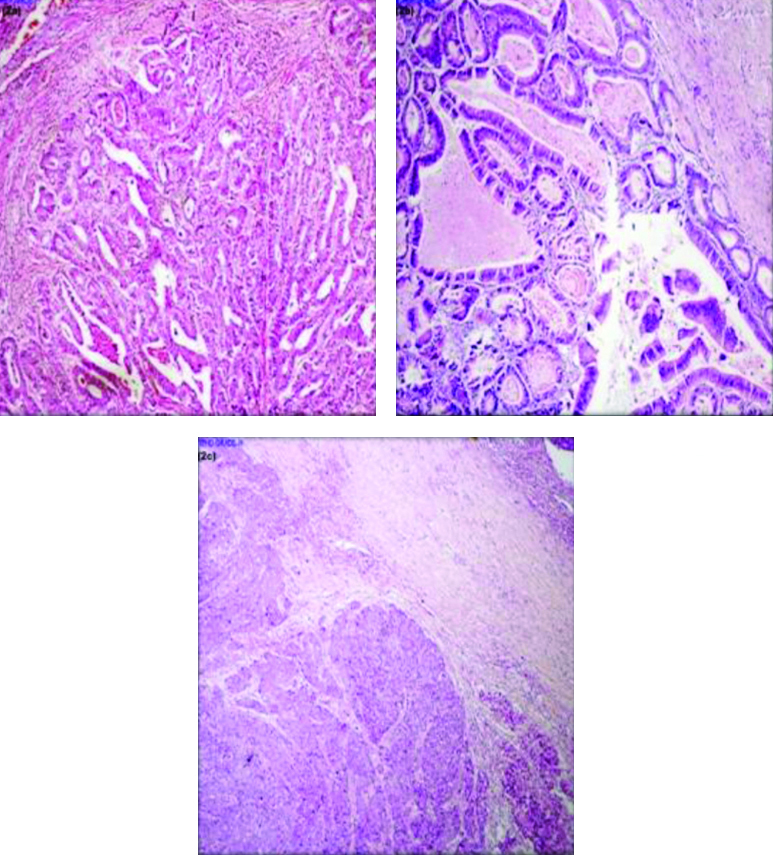
Institutional Ethical Committee (IEC no. JSSMC/PG/4700/2017-18) approval was taken. The clinical and histopathological data was retrieved and 3 μm thick sections were obtained from paraffin blocks and stained with H&E. For all prospective cases gross examination [Table/Fig-1] was done and slides were reviewed by two pathologists to observe the interobserver reproducibility who were blinded to the patient’s clinical information and the tumours were graded according to the recommendations of the WHO-2010 criteria’s. Well differentiated (>95% gland) [Table/Fig-2a], moderately differentiated (50-95% gland) [Table/Fig-2b], poorly differentiated (0-45% gland) [Table/Fig-2c], mucinous adenocarcinoma and signet ring cell carcinoma. Staging was done according to the eighth edition of the American Joint Commission on Cancer Staging System [8]. Finally, both pathologists arrived at a consensus, which was later subjected to PDC grading.
Gross specimen of colon showing ulceroinfiltrative growth.
a) Showing back to back arrangement of tumour cells in glandular pattern (H&E, x100); b) Showing tumour cells arranged in irregular glandular pattern (H&E, x100); c) Showing tumour cells arranged in solid sheets (H&E, x100).
PDC was evaluated in representative H&E stained slides at the advancing edge of the tumour and counting was done in the microscopic field of x20 objective lens (i.e., 1 mm microscopic field).
Poorly Differentiated Clusters
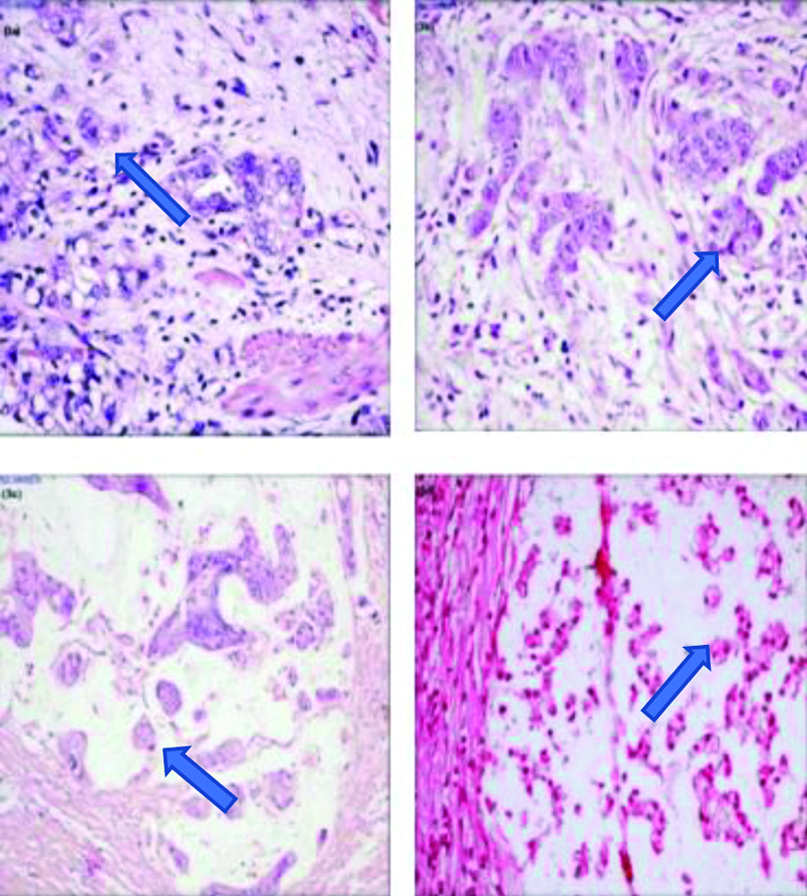
Poorly Differentiated Clusters (PDC) are defined as cancer cell clusters in the tumour stroma composed of ≥5 cancer cells and lacking a gland-like structure, regardless of the size of the cluster. The results were quantified as grades: Grade 1: <5 clusters [Table/Fig-3a]; Grade 2: 5-9 clusters [Table/Fig-3b]; Grade 3: ≥10 clusters. With regard to the assessment of mucinous carcinoma [Table/Fig-3c], malignant clusters without tubule formation with infiltration into the stroma and minimal extracellular mucin were classified as PDC’s. Also, clusters with invasive micropapillary pattern or signet ring cells [Table/Fig-3d] were accepted as PDC’s if they had clustering and no tubule formation.
a): Grade I PDC with 2 PDC clusters (H&E, x200); b): Grade II PDC with 9 PDC clusters (H&E, x200); c): Grade III mucinous adenocarcinoma showing >10 PDC clusters (H&E, x200); d): Grade II signet ring cell carcinoma showing >5 PDC clusters (H&E, x200).
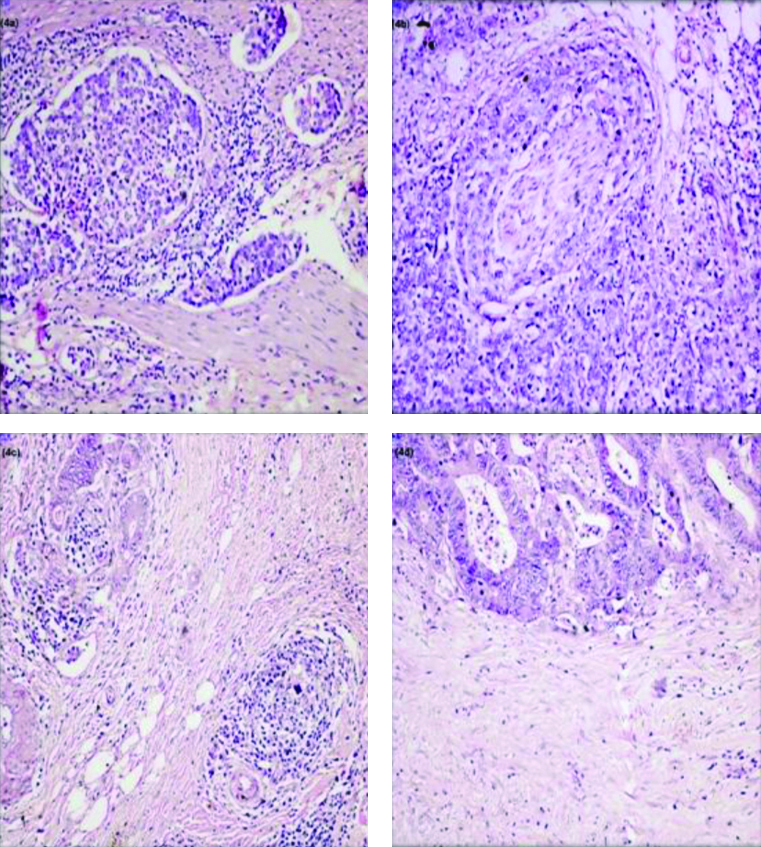
The following histopathological prognostic markers were studied: 1) Depth of invasion pT; Nodal Staging; 3) LVI, detection of cancer cells in peritumoural or intramural vessels [Table/Fig-4a]; 4) PNI, defined as tumour cells surrounding at least one-third of the nerve bundle and invading through the nerve sheath [Table/Fig-4b]; 5) The CLR, lymphoid aggregates embedded in a collagenous stroma within muscularis propria or pericolic fibroadipose tissue in advancing edge of CRC [Table/Fig-4c]; 6) DR, cancer-associated response of fibroblasts at the invasive front. Its presence or absence was mentioned with no further grading of DR [Table/Fig-4d]. In present study, CLR was just labelled as present or absent.
a): Showing Lymphovascular Invasion (LVI) (H&E, x200; b): Showing Perineural Invasion (PNI) (H&E, x200); c): Showing Crohn’s Like Lymphoid Reaction (CLR) (H&E, x 200); d): Showing desmoplastic stromal reaction (H&E, x400).
Statistical Analysis
The statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 22 computer program. All continuous variables were expressed as mean±SD (Standard Deviation) and categorical variables as percentage. The comparison of data from multiple groups was made by Pearson’s chi-square test and p-value was calculated. For interobserver variability independent sample t-test was used for calculating p-value. The p-value of <0.05 was considered statistically significant. Fleiss-Cohen’s weighted k statistics was also used for the assessment of inter-observer variability in tumour grading.
Results
Among 60 cases studied, the age of the patients ranged from 30 years to 82 years, with mean age of 59.13±13.85 years. Maximum number of patients belonged to 6th and 7th decades, amounting to total 30 cases (50%). A total of 28 (47%) were males and 32 (53%) were females with Male:Female ratio of 1:0.87. Commonest location of CRC was colon amounting to 36 cases (60%) followed by 15 cases (25%) in rectum and 9 cases (15%) in caecum [Table/Fig-5].
Representing age, sex, location, WHO and PDC grading in colorectal carcinomas.
| Feature | | G1 | G2 | G3 | Total | p-value |
|---|
| Total cases | | 6 | 29 | 25 | 60 | |
| Age | 30-82 years (mean-59.13±13.85) | | |
| Gender | Male | 28 (47%) |
| Female | 32 (53%) |
| M:F ratio | 1:0.87 |
| Site | Colon | 3 | 18 | 15 | 36 | 0.077 |
| Caecum | 1 | 1 | 7 | 9 |
| Rectum | 2 | 10 | 3 | 15 |
| WHO grading pathologist 1 | WD* | 3 | 3 | 1 | 7 | 0.007 |
| MD* | 1 | 23 | 14 | 38 |
| PD* | 0 | 0 | 4 | 4 |
| MAC* | 2 | 2 | 5 | 9 |
| SRCC* | 0 | 0 | 1 | 1 |
| WHO grading pathologist 2 | WD* | 3 | 4 | 1 | 8 | 0.003 |
| MD* | 1 | 23 | 13 | 37 |
| PD* | 0 | 0 | 5 | 5 |
| MAC* | 2 | 2 | 5 | 9 |
| SRCC | 0 | 0 | 1 | 1 |
| pT | T1 | 1 | 0 | 0 | 1 | 0.136 |
| T2 | 2 | 9 | 9 | 20 |
| T3 | 3 | 18 | 14 | 35 |
| T4a | 0 | 2 | 2 | 4 |
| pN# | N0 | 4 | 16 | 15 | 35 | 0.12 |
| N1 | 1 | 7 | 4 | 12 |
| N2 | 1 | 1 | 6 | 8 |
| LVI | Yes | 2 | 19 | 18 | 39 | 0.203 |
| No | 4 | 10 | 7 | 21 |
| PNI | Yes | 1 | 7 | 5 | 13 | 0.890 |
| No | 5 | 22 | 20 | 47 |
| DR | Yes | 3 | 19 | 16 | 38 | 0.770 |
| No | 3 | 10 | 9 | 22 |
| CLR | Yes | 6 | 21 | 21 | 48 | 0.247 |
| No | 0 | 8 | 4 | 12 |
*G1-PDC Grade 1, G2-PDC Grade 2, G3-PDC Grade 3; #There were five pN cases i.e lymphnodes
WD: Well differentiated; MD: Moderately differentiated; PD: Poorly differentiated; MAC: Mucinous adenocarcinoma; SRCC: Signet ring cell carcinoma; T: Tumour; N: Node; LVI: Lympho vascular invasion; PNI: Perineural invasion; DR: Desmoplastic Reaction, CLR: Chron’s like lymphoid reaction
WHO Grading
According to pathologist 1, 38 cases (63%) were reported as moderately differentiated followed by nine cases (15%) of mucinous adenocarcinomas, seven cases (11%) of well differentiated, four cases (7%) of poorly differentiated adenocarcinomas and one case (2%) of signet ring cell adenocarcinoma, while pathologist 2 reported 37 cases (61.7%) of moderately differentiated, eight cases (13.3%) of well differentiated and five cases (8.3%) of poorly differentiated while numbers of mucinous adenocarcinoma and signet ring cell carcinoma were same as pathologist 1 [Table/Fig-2a-c]. Finally, both pathologists arrived at a consensus which was later subjected to PDC grading [Table/Fig-5].
PDC Grading
According to PDC grading, maximum cases fell in the grade-II category followed by grade-III and grade-I. A total of 29 cases (48.3%) were of grade-II followed by 25 (41.7%) grade-III and only six cases (10%) of grade-I categories [Table/Fig-3a-d,5].
Histological Prognostic Markers
Lympho Vascular Invasion (LVI) was positive in 39 cases [Table/Fig-5]. Among them 19 cases were from grade-II, 18 cases from grade-III followed by two cases from grade-I. PNI was positive in 13 cases [Table/Fig-5], among them seven cases were from grade-II followed by five cases from grade-III and one case from grade-I. Forty eight cases (80%) showed CLR at the invasive front of the tumour. In rest 12 cases (20%) it was absent [Table/Fig-5]. Thirty eight cases (63%) showed DR [Table/Fig-4d] while rest 22 cases (37%) were negative for the same [Table/Fig-5]. DR was reported as either positive or negative but was not graded into mature, intermediate and immature forms in present study. In T3 category there were 35 cases (58.3%) followed by 20 cases (3.3%) of T2, four cases (6.7%) of T4a and one case of T1. There were no cases of T4b. The patients were grouped using the TNM staging for lymph node as pNx in five cases (8.3%) where lymph node status could not be assessed. There were 35 cases of pN0 (58.3%), four cases (6.7%) showed metastasis in 1 regional lymph node (pN1a), five (8.3%) cases in 2 to 3 regional lymph nodes (pN1b), three cases (5%) showed tumour deposits in the subserosa, mesentery or non-peritonealised pericolic region (pN1c), five cases (8.3%) showed metastasis in 4-6 regional lymph nodes (pN2a), three cases (5%) showed metastasis in 7 or more regional lymph nodes (pN2b).
Inter-observer Variability between two Pathologists
Inter-observer variability among pathologists was maximum in diagnosing poorly differentiated carcinoma followed by mucinous adenocarcinoma. There was a significant association between two pathologists with k-value of 0.852 and p-value of 0.001 which was statistically significant [Table/Fig-6].
Showing inter-observer variability between two pathologists.
| Association of observation by pathologist 1 and pathologist 2 | WHO grade | Percentage |
| Well differentiated | 100% |
| Moderately differentiated | 94.7% |
| Poorly differentiated | 75% |
| Signet ring cell carcinoma | 100% |
| Mucinous adenocarcinoma | 80% |
PDC with WHO Grading
Fleiss-Cohen’s weighted k statistics was used for the assessment of inter-observer variability in tumour grading. There was a significant association between PDC grading and WHO grading done by Pathologist-1 and Pathologist 2 with p-value of 0.007 (≤0.05) and 0.003 (≤0.05) respectively. Finally, both pathologists arrived at a consensus, which was later subjected to PDC grading [Table/Fig-5].
Discussion
One of the leading and most common causes of human cancer-related death is CRC [11]. The most widely used pathologic variables in histologic grading of CRC was framed by WHO and AJCC, but however it is very difficult to accurately define it due to difficulty in categorising variable degrees of differentiation which is one of the most distinctive characterstic features of this tumour. Till date, no single standard international criteria have been established for deciding whether grading should be diagnosed based on predominant pattern of differentiation or on considering the area of least differentiation [12]. Prognosis and treatment decisions of CRC’s are made primarily based on the extent of the disease, as defined by the TNM staging system. Unfortunately, a good number of CRC’s behave poor in spite of being labelled as low risk based on their TNM staging system [7]. Many studies have been taken up saying that PDC’s are independent prognostic factors in CRC’s and hence PDC assessment has a strong influence to increase prognostic accuracy and also to frame the treatment algorithms. In the present study, 60 resected cases of colorectal adenocarcinomas were graded according to the number of PDC’s as defined by Ueno H et al., and were analysed to determine the relationship of PDC grades with known histopathological prognostic factors [13]. Maximum number of patients was in the age group of 6th and 7th decades which was comparable with studies done by Kinoshita O et al., Ueno H et al., and Baressi V et al., [14-16]. There was female predominance with male to female ratio of 1:0.87 and was comparable with the study done by Kinoshita O et al., Colon was the commonest location followed by rectum and caecum [14].
Histological Grade and Inter-observer Variability
The issue of reproducibility in any tumour is always related to histopathologic diagnosis, and tumour grading is not an exception for this rule. The objective judgement of the WHO grading is therefore very problematic with significant inter-observer variability and the k-value of this 3-tiered WHO grading system was reported variously. Study done by Chandler IP and Houlston RS showed k-value of 0.35 [17], another study done by Ueno H et al., had k-value of 0.52 [13] and in the present study k-value was 0.852 with p-value of 0.001 which was statistically significant [Table/Fig-7].
Showing comparison between WHO and PDC grading ]12,13,16].
| Comparison of histological grade of the present study with other studies |
|---|
| Study | Total no. of cases | WD | MD | PD | MAC |
|---|
| Kim JW et al., [12] | 201 | 45 | 133 | 16 | 7 |
| Ueno H et al., [13] | 500 | 210 | 247 | 13 | 30 |
| Present study | Pathologist 1 | 60 | 7 | 39 | 4 | 9 |
| Pathologist 2 | 60 | 8 | 37 | 5 | 9 |
| Comparison of PDC grading with other studies |
| Study | G-I | G-II | G-III |
| Ueno H et al., [13] | 156 | 198 | 146 |
| Kim JW et al., [12] | 85 | 58 | 58 |
| Basressi V et al., [16] | 85 | 43 | 24 |
| Present Study | 6 | 29 | 25 |
| Comparison of PDC and WHO grading with present study and other studies |
| G-I | G-III | G-II | |
| Ueno H et al., [13] |
| WD* | 86 | 85 | 39 | p-value<0.0001 |
| MD* | 56 | 101 | 90 |
| PD* | 2 | 3 | 8 |
| MAC* | 12 | 9 | 9 |
| Kim JW et al., [12] |
| WD* | 35 | 6 | 4 | p-value<0.001 |
| MD* | 52 | 48 | 33 |
| PD* | 0 | 3 | 14 |
| MAC* | 3 | 1 | 3 |
*WD: Well differentiated; MD: Moderately differentiated; PD: Poorly differentiated; MAC: Mucinous adenocarcinoma
Poorly Differentiated Clusters (PDC)
Present study showed maximum cases falling into grade-II category which correlated with study done by Ueno H et al., while other studies showed maximum of grade-I followed by grade-II and grade-III [Table/Fig-7] [13].
PDC with WHO Grading of CRC
Present study proved that PDC grading has a more proportionate distribution of tumours compared to WHO grading. By WHO grading, 63% were moderately differentiated, 11% well differentiated, 7% poorly differentiated and rest 15% were mucinous adenocarcinomas. By PDC grading, G-II and G-III tumours increased to 48.3% and 41.7% respectively while G-I was only 10% with p-value of 0.003 which was statistically very significant. This also reflects biological aggressiveness of mucinous and signet ring cell carcinomas. Thus, PDC grading has strong ability to stratify patients into prognostic outcome compared to WHO grading. Present study with p-value of 0.003 is in concordance with the studies done by and Kim JW et al., Ueno H et al., with p-value<0.001 in all the three studies [Table/Fig-7] [12,13].
PDC Grading with Histopathological Prognostic Markers
In the present study, majority of cases were of pT3 (58.3%) followed by pT2 (33.3%) and pT4a (6.7%) which was comparable with study done by Baressi V et al., [10] while other studies showed pT3 followed by pT4a and pT2 except by Kim JW et al., [12] which showed pT3 followed by pT1, pT2, and pT4. Number of T3 and T2 cases was high in present study as most of the patient presented with advanced stage [Table/Fig-5]. Present study did not show any significant association between PDC and depth of invasion (pT) with p-value of 0.136 which was comparable to study done by Baressi V et al., [Table/Fig-5] [10]. In 5 (8.3%) out of 60 cases, lymph nodes could not be assessed in present study. The maximum cases were of N0 followed by N1 and N2 which was in concordance with other studies done by Barresi V et al., Kim JW et al., Ueno H et al., and Ueno H et al., [10,12,13,15]. There was near significant association between nodal staging and PDC grading with p-value of 0.12 [Table/Fig-5]. Patients with LVI and PNI tumours were more likely to have poor differentiation, high PDC grade, increased depth of invasion and advanced N category as found in present study with increased cases of PDC G-II and G-III tumours. One of the histological hallmark of invasive CRC is the response of fibrotic stroma induced by a tumour [18]. In present study, maximum cases with DR fell into G-II followed by G-III and G-I [Table/Fig-5]. CLR was associated with lower rate of loco regional recurrence, lesser distant metastasis, good cancer-specific and overall survival rate [19]. In present study, 48 out of 60 had CLR.
Limitation(s)
Main limitation of this study was that our institute is not cancer centre thus sample size was limited and this can be the reason for not finding significant association between PDC and histological predictors.
Conclusion(s)
Poorly differentiated clusters grading is a novel grading system and is more superior than WHO grading system in terms of inter-observer agreement and prognostic power. It is also proved that specific types like mucinous and signet ring cell carcinomas can be graded by the PDC grading system, as the current WHO system cannot be applied to all CRC types. Hence, PDC grading can be used as a new upcoming parameter having stability in distinguishing prognostic events and in planning therapy. There is a strong need to eliminate the inter-observer variability for the better prognosis and treatment of patients with CRC’s. PDCs is relatively a new parameter and is not much studied yet. This grading system deserves to be studied in a larger population of patients with CRC’s to further test its reproducibility, prognostic value and its significance in histological subtypes.
*G1-PDC Grade 1, G2-PDC Grade 2, G3-PDC Grade 3; #There were five pN cases i.e lymphnodes
WD: Well differentiated; MD: Moderately differentiated; PD: Poorly differentiated; MAC: Mucinous adenocarcinoma; SRCC: Signet ring cell carcinoma; T: Tumour; N: Node; LVI: Lympho vascular invasion; PNI: Perineural invasion; DR: Desmoplastic Reaction, CLR: Chron’s like lymphoid reaction