Dental caries is a complex multifactorial disease which affects a majority of people worldwide. Although preventable, it still remains a major problem [1].
Surface demineralisation may be prevented by applying surface remineralising agents over the surface. One of the most effective remineralising agents in caries prevention is fluorides and nanoparticles because of their anticariogenic characteristics [2].
An ecological imbalance between the tooth minerals and oral biofilm is seen as caries proceeds slowly. It is characterised by microbial activity, leading to fluctuations in the plaque pH because of bacterial acid production, buffering action from the saliva and the surrounding structures of the tooth [3]. Streptococcus mutans is most common bacteria associated with dental caries. The control of pathogenic bacteria, promotion of remineralisation and inhibition of demineralisation are two important aspects of the progress in prevention of caries [4,5].
Chitosan is widely used in drug delivery systems and tissue engineering. As it has a positive charge which accumulates on the cell walls of the bacteria, it therefore helps in giving a bactericidal and bacteriostatic property [6].
The use of SDF has gained a lot of attention in managing dental caries. Silver Diamine Fluoride (38%) is a colourless liquid containing silver particles and 38% (44,800 ppm) fluoride ion. It is composed of 25% silver, 8% ammonia, 5% fluoride and 62% water [7].
Acidulated Phosphate Fluoride Gel (APF) have been used in topical form for more than 50 years for prevention and control of dental caries. APF gel was introduced to dentistry in 1960 by Brudevold and his coworkers. This contains 1.23% of sodium fluoride, 0.1 M phosphoric acid, 50% Hydrofluoric acid and methyl cellulose/hydroxy ethyl cellulose [8].
Surface hardness of enamel refers to dental resistance against scratches, abrasion and indentation as well as resistance against permanent curvature and deformation at the time of force exertion and it fluctuates due to various reasons. The tooth decay plays a pivotal role in reducing enamel microhardness of the tooth; hence microhardness test is widely utilised to evaluate tooth hardness. pH cycling method is by creating acidic challenges which greatly helps in the simulation process of the mouth environment in the laboratory [9].
There is paucity in literature about evaluation of enamel microhardness and comparing APF gel, chitosan nanoparticles and SDF. Hence, this in-vitro study was carried out to evaluate and compare the efficacy of remineralising potential of APF gel, chitosan nanoparticle and SDF on microhardness of artificial carious lesions created on extracted teeth.
Materials and Methods
This was an in-vitro experimental study of Krishna Institute of Medical Sciences, Karad conducted during a period of two months (October and November 2019) at Mechanical Department of Rajarambapu Institute of Technology, Islampur Maharashtra. The study was approved by the Institutional Ethics Committee under the protocol number 0218/2018-19 Reference number (KIMSDU/IEC/08/2018). This study included three types of varnishes with different ingredients i.e. APF gel, chitosan nanoparticle and SDF to check the potential remineralisation of the teeth.
This study included 30 single rooted premolars which were extracted for orthodontic purposes. They were divided into groups of 10 each for each type of varnish applied. Decayed and decalcified teeth, including those with any white spot lesions were excluded from the study. After collection of teeth, they were kept in 0.5% Chloramine Tosyl Chloramide Sodium Salt solution to disinfect the teeth. The teeth were cleaned with ultrasonic scaler tip S2 (Guilin Woodpecker Company; China) for removing the debris prior to use and with a low-speed handpiece they were polished with prophylaxis paste using a polishing brush.
The samples were prepared with the help of a water-cooled diamond blade and a rapidly rotating air turbine; the teeth were then separated by giving a horizontal section from their roots through the Cementoenamel Junction (CEJ). Subsequently, each crown of the tooth was embedded in the acrylic resin with all sides of the crown, facing downwards inside the acrylic resin and the CEJ surface facing upwards [Table/Fig-1].
Crown of the tooth embedded in the acrylic resin.
After the resin acrylic polymerisation of each tooth was completed, in order to obtain nonslip measurements and a flat surface without the dentin coming out, the CEJ surfaces of the samples were flattened with 600 and 1,200 grit aluminium oxide abrasive papers.
Artificial caries like lesions were then created on the exposed enamel by suspending all teeth in an artificial caries system for five days and then the solution was kept at a temperature of 37°C. Artificial caries solution was prepared by mixing 5 litres of distilled water, 2.205 grams of Calcium Chloride (CaCl2) with 2.041 grams Potassium dihydrogen phosphate (KH2PO4). To this 10 mL methylhydroxidiphosphonate (MHDP) solution was added. Then, 14.3 mL acetic acid (CH3COOH) (50 mM) and 10 M potassium hydroxide (KOH) were added to titrate the solution at pH 4.95 [10].
After five days the teeth were removed from the artificial caries system. Then baseline microhardness for enamel was determined by vickers hardness indentation machine.
The baseline microhardness of teeth was measured and recorded by applying 200 gm force for a dwell time of 15 seconds [11]. The experimental varnishes 1.23% APF Gel (Fluorovil), 0.2% chitosan nanoparticle (Nano Research Elements) and 38% SDF (FAgamin) as per manufacturer’s instructions were then applied on enamel surface of the teeth for four minutes. Low molecular weight chitosan was dissolved at a concentration of 3 mg/mL in 0.5% acetic acid solution, then raised to pH 4.6 with 10 Normality of Sodium Hydroxide (NaOH) and filtered the solution using filter membrane. These varnishes were slowly cleaned from the surface of treated samples with periodontal curette after 24 hours to synchronise the loss of varnish from the enamel surface in oral cavity.
To create laboratory conditions similar to the mouth, the samples were then entered into pH cycle. pH cycle comprised three hours of immersion in the demineralising solution, 20 hours of immersion in remineralising solution followed by distilled water for 30 minutes. Each cycle was performed for 24 hours. This was done for 28 days.
Thus, for three hours, samples were placed in a demineralisation solution. The demineralising solution included 2.2 mM CaCl2, 2.2 mM NaHPO4, 0.05 M acetic acid, callibrated with 1 mL KOH at pH=4.5. The samples were then immersed for 30 minutes, in distilled water.
Thereafter, for 20 hours samples were left in a remineralisation solution followed by washing. Further samples were immersed in distilled water for 30 minutes. The remineralising solution constituted 1.5 mM CaCl2, 0.9 mm NaHPO4, KCl 0.15 mM with pH=7.0.
The demineralisation and remineralisation solutions were changed on the fourth day of pH cycling so that concentration of calcium and phosphate ions in solution would not affect the results [12].
The pH cycle was completed after 28 days. Thus, 28 pH cycles were performed. Teeth samples were removed from remineralisation solution and then enamel samples were subjected for microhardness testing by Vickers hardness indentation machine (Future-Tech Corp FM-700, Tokyo, Japan). The summary of methodology has been shown in flowchart [Table/Fig-2].
Flowchart showing the summary of methodology.
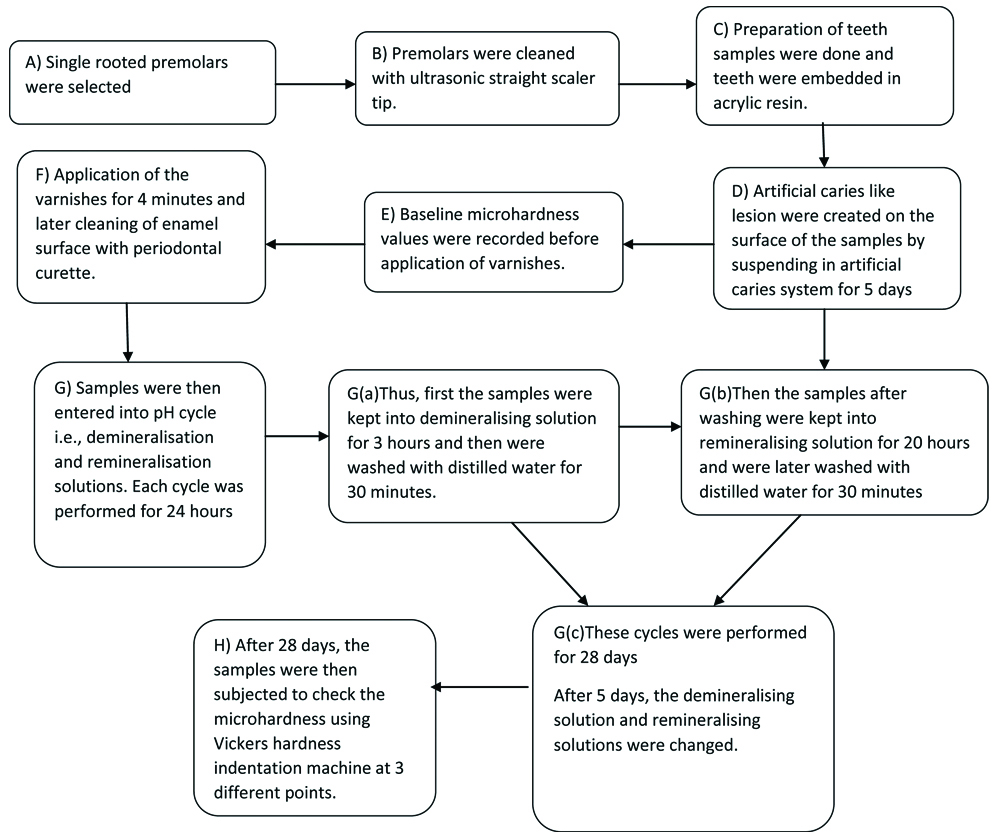
Data was then recorded for statistical test. Enamel microhardness was measured at three different points i.e., it was measured at occlusal, middle, cervical points and mean values were obtained as Vickers Hardness Number (VHN). The data was recorded and subjected to statistical analysis.
Statistical Analysis
Enamel microhardness was measured at three different points i.e., occlusal, middle, cervical points and the average value was calculated and recorded as VHN. The data i.e., mean, standard deviation, intergroup and intra-group comparison were then recorded, tabulated and Software version for Kruskal-Wallis Test: - XLSTAT using Kruskal-Wallis Test and Wilcoxon Test to find out if there was a significant difference. p<0.05 was considered as statistically significant.
Results
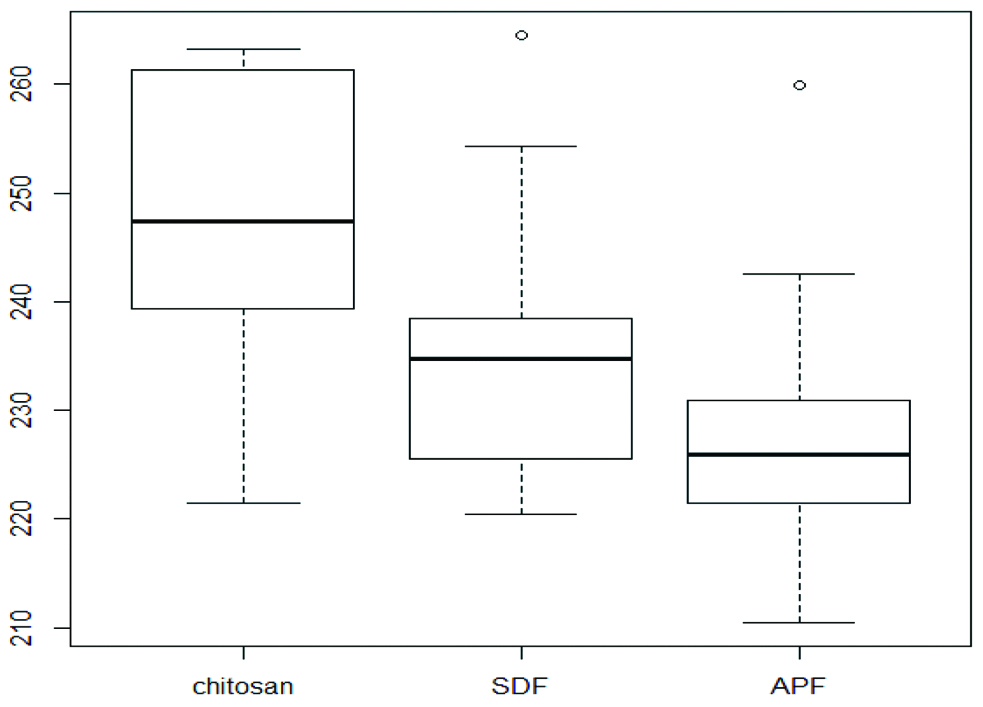
The inter group comparison of varnishing material was done using Kruskal-Wallis Test. [Table/Fig-3] shows intergroup comparison pre and post-varnish application. There was no statistical difference between the groups before the application of varnishes with p=0.85. The mean values of VHN in chitosan group was 246.8±14.9, for SDF it was 235.9±14.1 and for APF gel it was 229±13.6. While there was a statistically significant difference in the groups after application of varnish with p=0.035.
Results obtained for inter group comparison using Kruskal-Wallis Test.
| Hardness | Groups | n | Mean | Median | SD | df | Kruskal-Wallis Test | p-value |
|---|
| Before intervention | Chitosan | 10 | 216.4 | 212.4 | 12.9 | 2 | 0.32208 | 0.851 |
| Silver diamine fluoride | 10 | 217.9 | 215.2 | 10.0 |
| APF gel | 10 | 217.2 | 217.3 | 11.6 |
| After intervention | Chitosan | 10 | 246.8 | 247.5 | 14.9 | 2 | 6.6776 | 0.035* |
| Silver diamine fluoride | 10 | 235.9 | 234.8 | 14.1 |
| APF gel | 10 | 229.0 | 225.9 | 13.6 |
Here, N: Sample number; SD: Standard deviation; df=: egrees of freedom and Before intervention signifies after artificial caries creation and After intervention means after varnish application and pH cycle
Further, the intra group comparison of varnishes was done using Wilcoxon Signed Rank Test. [Table/Fig-4] showed that all three materials had a significant difference in VHN values pre and post-varnish application with p-value being <0.001 statistically highly significant for chitosan and p=0.005 for SDF and APF gel.
Results obtained for intra-group comparison using Wilcoxon Signed Rank Test.
| Groups | n | | Mean | Median | SD | p-value |
|---|
| 1. Chitosan | 10 | Before intervention | 216.4 | 212.4 | 12.9 | 0.001** |
| 10 | After intervention | 246.8 | 247.5 | 14.9 |
| 2. Silver diamine fluoride | 10 | Before intervention | 217.9 | 215.2 | 10.0 | 0.005* |
| 10 | After intervention | 235.9 | 234.8 | 14.1 |
| 3. APF gel | 10 | Before intervention | 217.2 | 217.3 | 11.6 | 0.005* |
| 10 | After intervention | 229.0 | 225.9 | 13.6 |
Here, N: Sample number; SD: Standard deviation; and before intervention signifies after artificial caries creation and After intervention means after varnish application and pH cycle
[Table/Fig-5] shows that after intragroup comparison between all three materials, chitosan nanoparticle showed highest increase in microhardness than SDF and APF gel with median value of 247.5.
Box plot of VHN for various varnishes.
VHN: Vickers Hardness Number
Discussion
It is important to evaluate any changes in the surface layer of enamel as it plays a key role in the process of tooth decay. In this study, surface microhardness was determined by using Vickers hardness number. High accuracy and quantitative measurement capability is the advantage of this method [13]. Another advantage is the possibility of application of force with different sizes and repeated measurement of hardness of specimens within a specific time which can be done in this method [14]. The present study results coincided with study carried out by Kumar P et al., and Murakami C et al., conducted a comparative study on the microhardness and surface morphology of tooth enamel with Er Cr:YSGG laser (Erbium, chromium-doped yttrium, scandium, gallium and garnet) irradiation and APF gel. They observed significantly higher increase in microhardness (p<0.05*) when APF gel was used prior to Er, Cr:YSGG laser irradiation [15]. Whereas, study carried out by Murakami C et al., concluded that APF gel when compared with Duraphat (1 mL suspension contains 50 mg sodium fluoride {5%w/v}) in all permanent teeth groups showed significant increase in enamel hardness from the second to the seventh day of their study (p<0.001**) [16].
Results were determined with the study conducted by Villena R.S. Study suggested that the application of APF for either 1 or 4 minutes was equally effective to increase the concentration of enamel in fluoride and consequently reducing enamel demineralisation although the difference was not significant. They concluded that it helps in increase of microhardness of enamel [17].
The results of the study by Delbem AC were steady with present study in case of APF gel. The authors concluded that APF gel compared to NaF significantly increased microhardness with p<0.005* [18].
Firouzmandi M et al., concluded that the adjunctive application of SDF and Proanthocyanidin had a similar remineralising effect to that of SDF on caries with a significant difference, p-value 0.005* [19]. Thus, supporting present results in respect to Silver Diamine Fluoride.
Similarly another study carried out by Shah S et al., showed similar results with silver Diamine fluoride. They concluded that in-vivo application of SDF on enamel significantly decreased S. mutans counts as compared to fluoride varnish and APF gel. Thereby, increasing the microhardness of the tooth [20].
Huang WT et al., explained that the cariostatic mechanism of SDF treatment is associated with the formation of fluoride and silver compounds, antibacterial properties, and anticollagen degradation efficacy which help in arresting the caries and thus leading to increase in microhardness [21].
In case of chitosan, results of present study were alike with the study carried Tachaboonyakiat W et al., and Pimenta JA et al., explained that when chitosan interacted with gram positive and gram negative bacteria a film acts as an effective antibacterial barrier on the tooth surface because of its broad-spectrum antimicrobial activity which helps to significantly increase the surface microhardness [22].
While Pimenta JA et al., explained that 0.2% chitosan nanoparticle was equally effective to 15% EDTA and 10% citric acid against microhardness reduction (p>0.05) [23].
Study conducted by Arnaud TM et al., proved that enamel of teeth sections treated with chitosan nanoparticle and then immersed in a demineralisation solution showed significantly higher surface hardness. For chitosan concentrations of 2.5 g/mL and 5.0 g/mL, the penetration was up to the Dentin-Enamel Junction (DEJ) than untreated enamel of teeth section which coincided with the results seen in the current study with respect to chitosan [24].
The results of the present study were consistent with those of Ruan Q et al., who showed that enamel surface treated with chitosan-amelogenin hydrogel before and after demineralisation procedures in the pH cycling method showed repair of the artificial caries and thus concluded that Chitosan-Ameleogenin Hydro gel (CS-AMEL) hydrogel offered potential benefits in the prevention and treatment of damaged enamel [25].
Limitation(s)
Since this study was carried out in laboratory environment, it was not possible to mimic the in-vivo characteristics like the speed flow and rate of saliva. Hence further clinical studies are indicated. Moreover, in-vitro studies are faced with the limitation of laboratory and clinical set up errors.
Conclusion(s)
Within the limitation of the study, we conclude that chitosan, SDF and APF gel significantly increased enamel microhardness and remineralisation. Chitosan showed highest increase in microhardness of teeth followed by SDF and APF Gel. Further studies should be carried out to substantiate the findings of this study.
Here, N: Sample number; SD: Standard deviation; df=: egrees of freedom and Before intervention signifies after artificial caries creation and After intervention means after varnish application and pH cycle
Here, N: Sample number; SD: Standard deviation; and before intervention signifies after artificial caries creation and After intervention means after varnish application and pH cycle