Testicular Lymphoma: A Rare Entity
Soumya Mondal1, Eeshansh Khare2, Dilip Kumar Pal3
1 Assistant Professor, Department of Urology, IPGME&R, Kolkata, West Bengal, India.
2 Postdoctoral Trainee, Department of Urology, IPGME&R, Kolkata, West Bengal, India.
3 Professor and Head, Department of Urology, IPGME&R, Kolkata, West Bengal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Dilip Kumar Pal, Professor and Head, Department of Urology, IPGME&R, Kolkata-700020, West Bengal, India.
E-mail: drdkpal@yahoo.co.in
Testicular malignancy in old age men should be carefully studied because of low rate of incidence. Incidence of testicular lymphomas have been shown to be nearly 9% among all testicular malignancies and 1-2% of all lymphomas found in human body. The most common hisopathological pattern among lymphomas is B-cell type and of these diffuse large B-cell subtype is commonest; however, less commonly, Burkitt’s lymphoma, anaplastic lymphoma or Hodgkin’s lymphoma may primarily involve the testis. Testicular lymphoma is rare whether as a primary or as a secondary origin. Diffuse Large B-Cell Lymphomas (DLBCL) is the most common histopathological type if primary in origin; however aggressive patterns like, Burkitt’s lymphoma, are more common when there is secondary involvement of testis. Stage of the lymphoma (Ann Arbor staging) determines the prognosis of the disease. Other prognostic factors include presence of B symptoms and International Prognostic Index (IPI) score, involvement of spermatic cord and other testis. Immunohistochemistry should be done after histopathological diagnosis for effective treatment of variants of testicular lymphoma. High inguinal orchiectomy along with chemotherapy with or without radiotherapy is current treatment of choice for these tumours. In this article, the case of testicular lymphoma is presented in a 61-year-old male patient, clinical presentation, investigations and surgical and chemotherapeutic management with discussion with respect to previous clinical studies. In this case report, patient presented with swelling of left scrotum for which he underwent the radiological and biochemical investigations. He was further planned for frozen section biopsy. The histopathological and immunohistochemical analysis was suggestive of DLBCL, as final diagnosis.
Immunohistochemistry, Non-hodgkin’s lymphoma, Orchiectomy
Case Report
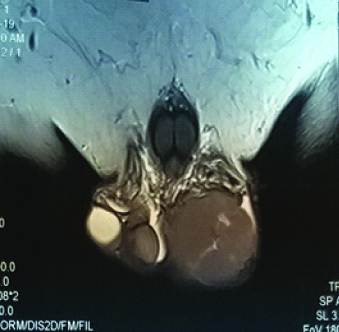
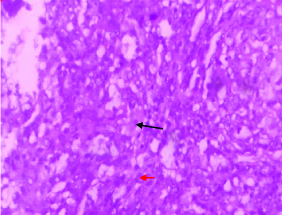
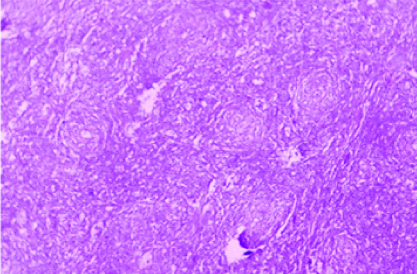
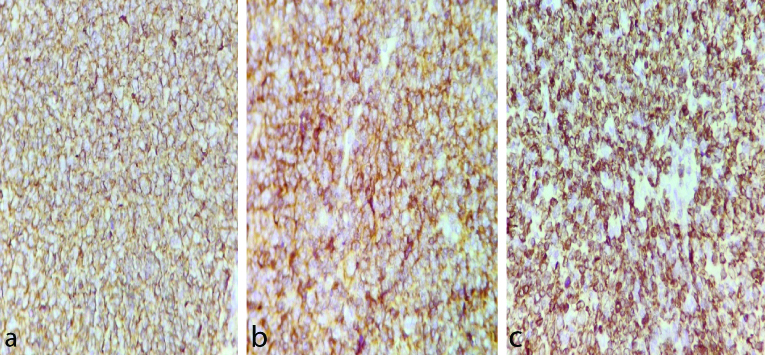
A 61-year-old man presented with swelling on left side of scrotum for last three months in our Outpatient Department. The swelling was painless, hard with palpable spermatic cord. Local examination revealed enlargement of left testis; it measured around 6×5 cm. There was no local rise of temperature or tenderness. His IPI score [1] was one out of maximum score of five (age > 60 years). There was no history of fever, weight loss and sweating in this patient. Past history was insignificant. Testicular ultrasound showed enlarged left testis with hypoechoic Space Occupying Lesion (SOL). Contrast Enhanced Computed Tomography (CECT) whole abdomen showed left testicular SOL measuring 6×5×4cm. Magnetic Resonance Imaging (MRI) of testis showed 6×4.6×4 cm SOL in left testis [Table/Fig-1]. X-ray chest was non-contributory. Routine blood parameters were within normal range. Biochemical analysis showed Lactate Dehydrogenase (LDH): 309 U/L; Beta Human Chorionic Gonadotropin (hCG): <2 ng/mL; and alpha fetoprotein: 1.7 ng/mL, all within normal range. After making a provisional diagnosis of left testicular SOL probably a malignancy, and differential diagnosis of long standing hydrocoele, patient was planned for exploration of the left testis with high inguinal route with a frozen section biopsy, which suggested a malignant lesion. The final histopathology revealed a tumour composed of monotonous population of atypical lymphoid cells with irregular nuclei and inconspicuous nucleoli with scant cytoplasm [Table/Fig-2a,2b]. Intertubular proliferation of these cells was also noted. Attached spermatic cord showed no involvement by tumour cells. Other differential diagnosis were follicular lymphoma, peripheral T cell lymphoma and anaplastic lymphoma and Hodgkin’s lymphoma, so immunohistochemistry was done for finalising the diagnosis and thus, Immunohistochemistry under high power (x400) showed lesional cells positive for Cluster of Differentiation-20 (D20), CD10, B-cell lymphoma-2 (Bcl-2) [Table/Fig-3a-c], Bcl-6 and Multiple Myeloma Clusters Of Differentiation-1 (MUM1) and negative for CD5 and Terminal deoxynucleotidyl Transferase (TdT); 20% lesional cells expressed c-myc; CD3 and CD5 mark background for lymphoid cells suggesting DLBCL. Panels were selected on the basis of afforementioned differential diagnosis. Patient was then referred to radiotherapy department where he was put on rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone (R-CHOP) regimen and patient is doing well since then on regular follow-up since six months.
MRI cut showing left testicular Space Occupying Lesion (SOL).
Showing scant cytoplasm (red arrow) and atypical lymphoid cells (black arrow); H&E 400X.
Showing monotonous atypical lymphoid cells;H&E(10X).
a) Slide showing CD20 marker membrane positivity (with x400 high power magnification); b) Slide showing CD10 marker membrane positivity (with x400 high power magnification); Slide showing Bcl 2 marker nuclear and membranous positivity (with x400 high power magnification).
Discussion
Testicular swelling in old age group must be given special importance and should be examined thoroughly to rule out any malignancy as these swellings are mostly painless with few general systematic symptoms. This may be one of the reasons for testicular tumours in old age to be diagnosed late in developing countries. Very few case reports have been published for testicular lymphoma and here patient was presented with testicular swelling in the age of 61 years and finally diagnosed as a case of testicular lymphoma. Though testicular lymphomas are rare yet they show relapse in 12% cases [2]. Primary Testicular Lymphoma (PTL) is the most common type of testicular cancer in men over the age of 60 years [3], and represents 1% of all Non-Hodgkin’s Lymphomas and involvement of bilateral tumours in 38% of cases [4]. DLBCL make up 90% Primary Testicular Lymphomas (PTLs) [5]. The most common clinical features are painless testicular swelling; fever, weight loss, night sweating (B-symptoms) and fatigue and loss of appetite can also be seen [6]. Though intratubular germ cell tumours are the precursor lesion of most testicular tumours yet it is not present in PTL [7].
The Ann Arbor staging is commonly used for PTL [8]. Ann arbor staging according to medical literature is as follows: Stage I: Involvement of single lymph node region or a single extralymphatic site. Stage II: Involvement of two or more lymph node region on the same side of diaphragm or with localised involvement of extralymphatic site. Stage III: Involvement of lymph node regions on both sides of diaphragm or with localised involvement of extralymphatic site or spleen or both and Stage IV: Disseminated involvement of one or more extralymphatic site with or without associated lymph node involvement. PTL can also be staged according to the Nordic Lymphoma Group as: Stage I: Unilateral testis involvement with or without epididymis or cord involvement; Stage II: Abdominal and pelvic lymph node involvement; and Stage III-IV: Distant metastasis [9]. Lymphomas are identified as hypoechoic lesions on scrotal ultrasound as opposed to homogeneous and hyperechoic normal testicular tissue. The immunohistochemical examination report showing presence of myc and Bcl-2 is considered as an adverse prognostic sign [10]. Management of stage I and II cases include high inguinal orchiectomy R-CHOP and may be followed by scrotal radiotherapy and systemic chemotherapy [11]. Relapse is common if adjuvant therapy is not given after surgery even in stage I tumours. In stage II cases, radiotherapy to localised lymph nodes can prevent nodal relapse. However, in stage III-IV diseases, systemic chemotherapy, scrotal radiotherapy, and intrathecal chemotherapy are possible options.
In present case, patient (stage I) received R-CHOP regimen after histopathological confirmation of testicular lymphoma after high inguinal orchiectomy. The clinical stage and histological grade are most important for prognostic significance [12]. Poor prognostic features for testicular lymphoma are larger primary tumour, involvement of epididymis and spermatic cord and involvement of bilateral testis, vascular invasion, old age, high LDH levels, presence of B-symptoms (fever without any infection; drenching night sweats; weight loss of >10% over six months), high IPI score (age >60 years; LDH >1x normal value; Eastern Cooperative Oncology Group (ECOG) performance status 2-4; clinical stage III and IV and extranodal disease > one localisation) [13-15], whereas good prognostic features are young age, localised and small sized tumour, low histological grade and no involvement of epididymis or spermatic cord.
DLBCL should be distinguished from the other testicular lymphomas; for example, T-cell lymphoma and Hodgkin’s lymphoma, because of the different management options. Most of patients with testicular large cell lymphoma have a poor prognosis. There is poor survival in cases of relapse. Treatment failure usually occurs within one to three years after the initial therapy.
Conclusion(s)
Testicular non-hodgkin’s lymphomas are highly aggressive cancers. Low incidence rate of the disease and tumour nature makes it hard to standardise the management after orchiectomy. Involvement of contralateral testis and central nervous system involvement should always be kept in mind during the diagnosis, treatment and follow-up of these patients. IPI score should be determined in such patients and B-symptoms should be determined. Immunohistochemical techniques are mandatory for the specific management of these tumours. Treatment of such tumours should be based on stage of the tumour and should be individually planned and patient should be regularly followed for recurrence.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jul 26, 2020
Manual Googling: Sep 26, 2020
iThenticate Software: Oct 28, 2020 (18%)
[1]. Sehn L, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOPBlood 2006 109:1857-61.10.1182/blood-2006-08-03825717105812 [Google Scholar] [CrossRef] [PubMed]
[2]. Zouhair A, Herrmann E, Ugurluer G, Gaye P, Mirimanoff R, Ozsahin M, Primary testicular lymphomaSwiss Med Wkly 2010 :14010.4414/smw.2010.1307620872294 [Google Scholar] [CrossRef] [PubMed]
[3]. Zucca E, Conconi A, Mughal TI, Sarris AH, Seymour JF, Vitolo U, Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the international extranodal lymphoma study groupJ Clin Oncol 2003 21(1):20-27.10.1200/JCO.2003.11.14112506165 [Google Scholar] [CrossRef] [PubMed]
[4]. Ferry JA, Harris NL, Young RH, Coen J, Zietman A, Scully RE, Malignant lymphoma of the testis, epididymis, and spermatic cordThe Am J Surg Pathol 1994 18:376-90.10.1097/00000478-199404000-000068141430 [Google Scholar] [CrossRef] [PubMed]
[5]. Salem YH, Miller HC, Lymphoma of genitourinary tractJ Urol 1994 151:1162-70.10.1016/S0022-5347(17)35204-7 [Google Scholar] [CrossRef]
[6]. Akdemir F, Okulu E, Kayıgil Ö, Orhun HS, Primary testicular lymphoma: A case reportJ Urol Surg 2015 3:150-52.10.4274/jus.317 [Google Scholar] [CrossRef]
[7]. Kim HS, Primary testicular diffuse large B-cell lymphoma: A case report focusing on touch imprint cytology and a non-germinal center B-cell-like phenotypeExperimental and Therapeutic Med 2013 6:33-36.10.3892/etm.2013.109123935714 [Google Scholar] [CrossRef] [PubMed]
[8]. Cheah CY, Wirth A, Seymour JF, Primary testicular lymphomaBlood 2014 123:486-93.10.1182/blood-2013-10-53065924282217 [Google Scholar] [CrossRef] [PubMed]
[9]. Ergün O, Koşar A, Baş E, Bircan S, Alanoğlu EG, Primary testicular lymphoma a case report and efficacy of chemotherapy combined with rituximabTurkiye Klinikleri J Med Sci 2011 31:748-51.10.5336/medsci.2009-12479 [Google Scholar] [CrossRef]
[10]. Wang Q, Zheng D, Chai D, Wu S, Wang X, Chen S, Primary testicular diffuse large B-cell lymphomaMedicine 2020 99(12):e1946310.1097/MD.000000000001946332195944 [Google Scholar] [CrossRef] [PubMed]
[11]. Reyes F, Lepage E, Ganem G, Molina TJ, Brice P, Coiffier B, ACVBP versus CHOP plus radiotherapy for localised aggressive lymphomaN Engl J Med 2005 352:1197-205.10.1056/NEJMoa04204015788496 [Google Scholar] [CrossRef] [PubMed]
[12]. Mazloom A, Fowler N, Medeiros LJ, Iyengar P, Horace P, Dabaja BS, Outcome of patients with diffuse large B-cell lymphoma of the testis by era of treatment: The M. D. Anderson Cancer Center experienceLeuk Lymphoma 2010 51:1217-24.10.3109/1042819100379335820443676 [Google Scholar] [CrossRef] [PubMed]
[13]. Sachs BA, Shahab N, Kaplan PA, Doll DC, Haider S, Conditions suggesting lymphomaJ Clin Oncol 2005 23:3843-44.10.1200/JCO.2005.04.16615923577 [Google Scholar] [CrossRef] [PubMed]
[14]. Chen B, Cao DH, Lai L, Guo JB, Chen ZY, Huang Y, Adult primary testicular lymphoma: Clinical features and survival in a series of patients treated at a high-volume institution in ChinaBMC Cancer 2020 20:22010.1186/s12885-020-6711-032171265 [Google Scholar] [CrossRef] [PubMed]
[15]. Guler Y, Ucpinar B, Erbin A, Diffuse large b-cell lymphoma of testis: A case report and current literature reviewFolia Medica 2020 62:200-03.10.3897/folmed.62.e4787432337891 [Google Scholar] [CrossRef] [PubMed]