Some chemotherapeutic agents like Anthracyclines and targeted therapy drugs like Trastuzumab are known cardio-toxic agents and further increase the risk of cardiac morbidities in breast cancer patients [9,10]. The major coronary events are seen to increase proportionally with increase in Mean Heart Dose, i.e., 7.4% for every 1 Gray of radiation [4]. Cardiac complications of radiotherapy are generally manifested much later after treatment, i.e., at least 10 years, so it is difficult to diagnose [10]. With advent of newer chemotherapeutic drugs, better surgical techniques and instruments and more advanced radiotherapy machines, the survival and life expectancy of breast cancer patients are increasing exceptionally after treatment completion. So, the cardiac complications of radiotherapy have now emerged as a great challenge for the radiation oncologist [11]. The aim of the present study was to correlate MHD with the mean heart dose received by heart in carcinoma breast patients receiving radiotherapy.
Materials and Methods
The Prospective observational study was approved by Institutional Ethics Committee Acharya Harihar Regional Cancer Centre (vide letter number 041-IEC-AHRCC). The sampling method used was purposive sampling. Out of all breast cancer patients who attended department of radiotherapy Acharya Harihar Regional Cancer Centre from January 2017 to January 2019.
Inclusion criteria: Ninety patients of histopathologically proven non-metastatic carcinoma of breast (both right-sided and left-sided breast cancer), who had undergone either Modified Radical Mastectomy (MRM) or Breast Conservation Surgery (BCS) with indications for adjuvant radiotherapy and who gave consent were included in the prospective observational study.
Exclusion criteria: Patients with history of prior surgery or radiotherapy to either breast and patients requiring internal mammary chain radiation were excluded from the study.
All patients were treated using 3DCRT technique. Every patient was taken for CT simulation in free breathing state. Each of them was immobilised and placed on an inclined breast board in supine position. Both of the arms of patients were abducted >90° and head tilted to opposite of the diseased side. Radio-opaque wires were utilised to mark postoperative scar. Clinical extent of opposite breast was found out by palpation and fiducial markers were placed on diseased side at 2 cm below the clinical extent of opposite breast. After this setup, patients were scanned on CT-simulator and 5 mm slices taken from mid-neck to mid-abdomen. The CT data sets obtained from the CT simulator were imported into Oncentra Treatment Planning System (TPS).
After CT simulation, clinical target volume contoured including ipsilateral breast/chest wall which was based on CT image and clinical border marked by fiducial markers. The Organs at Risk (OARs) were contoured which included the bilateral lungs, heart and opposite breast. Both lungs were contoured as organ at risk with exclusion of hilum and trachea [12]. Heart was contoured including pericardium which started from immediately inferior to left pulmonary artery and ended distally in the region where heart approached the diaphragm [12]. Planning target volume was created by giving 5 mm margin around the clinical target volume [Table/Fig-1,2] [13].
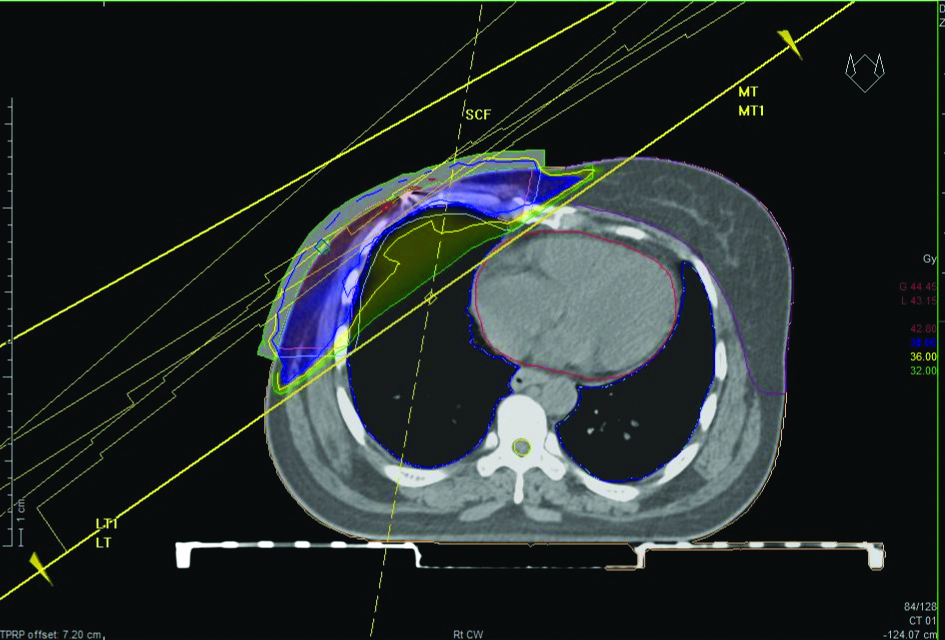
Free breathing CT scan of a patient with left-sided breast cancer postMRM. Clinical target volume, planning target volume and organ at risk contoured and parallel opposed treatment plan created. Light Blue- planning target volume, red- clinical target volume, pink- heart, orange- right breast, yellow- spinal cord.
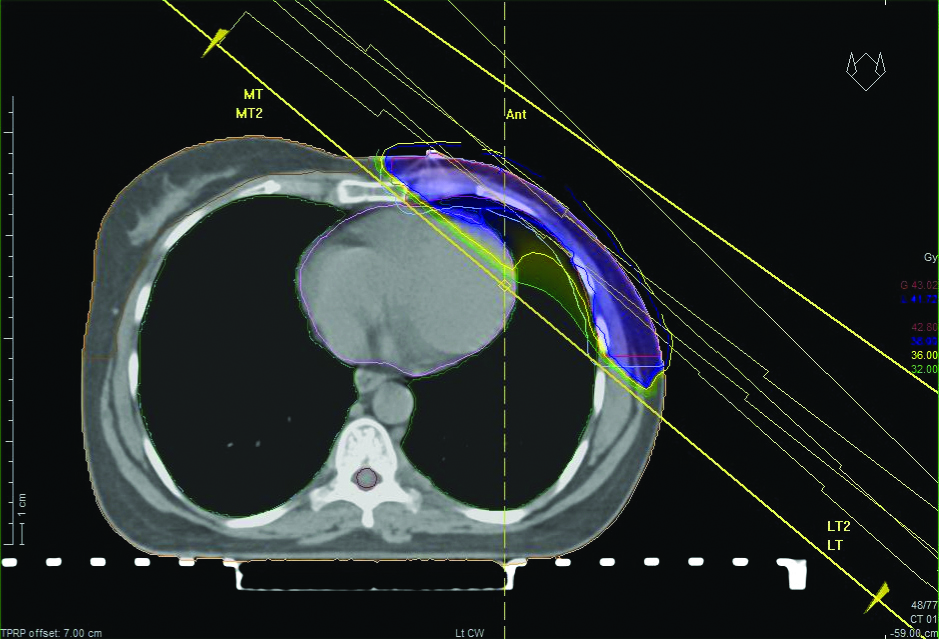
Free breathing CT scan of a patient with right-sided breast cancer postmodified radical mastectomy. Clinical target volume, planning target volume, organ at risk contoured and parallel opposed treatment generated. Light Blue- planning target volume, pink- clinical target volume, red- heart, violet- left breast, yellow- spinal cord.
For BCS, clinical target volume included all residual mammary tissues with cranial border head of clavicle, caudal border 20 mm inferiorly to breast fold, the mid sternal line as medial border and mid axillary line as lateral border. For mastectomies, caudal margin was defined based on fiducial marker placed 2 cm below extent of opposite breast which was established by palpation of contralateral breast [14]. Guidelines published by Radiation Therapy Oncology Group (RTOG) were followed for contouring of target volume and OAR [14].
After delineation of clinical target volume, planning target volume and organ at risk, CT data were transferred to the TPS (Monaco). All patients received hypo fractionated regimen of 40 Gy in 15 fractions, 2.67Gy per fraction, five fractions per week, Monday through Friday for three such weeks [11].
Treatment Planning
Based on the contouring of free breathing CT scan as described above, treatment plans were generated. Parallel opposed tangential treatment plans were created. Multi-leaf collimators were utilised in generating the plans. Field-In-Field (FIF) technique was used for treatment plan generation and wedges were used to avoid hotspots exceeding 110% so that dose homogeneity was ensured. Without compromising target volume dose, dose to OAR was maintained as low as possible [13]. Plans were approved when a minimum of 90% of prescribed dose covered by 90% of target volume as per the institutional protocol. Dose prescribed was 40Gy in 15 fractions at the isocenter in all cases [11]. Dose and volumetric data was obtained from dose volume histograms created by TPS and assessed for plan approval:
Heart:
Maximum Heart Distance (MHD): Defined as maximum distance within the radiation field between the anterior edge of heart contour and posterior border of tangential field [15].
Mean Heart dose: less than 26Gy [16] (also as per institutional protocol)
V5 (Volume proportion of heart receiving 5Gy or higher dose) ≤ 40% [17]
V20 (Volume proportion of heart receiving 20Gy or higher dose) ≤20% [17]
Lungs: V20 (Volume proportion of lung receiving 20Gy or more) ≤ 45% [17]
Contralateral breast: V5 (volume proportion of contralateral breast receiving 5Gy) less than 15% [17]
Target: PTV90- 90% of prescribed dose covered by percentage of target volume
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 21. The Spearman’s Rho test was used for correlation of MHD with Mean heart dose. The Mann-Whitney U test was used for comparing means of MHD in left-sided and right-sided breast cancer. The Independent t-test was used for comparing means of Mean heart dose in left-sided and right-sided breast cancer. The p-value <0.05 (marked with) was considered as statistically significant.
Results
In this study, 90 eligible Breast cancer patients were taken postsurgery for radiotherapy; among them 60 patients were left-sided and 30 patients were right-sided. The mean age of patients was 46 (SD±11.3) years. The average values of dose received by heart and MHD are summarised in [Table/Fig-3]. As shown in [Table/Fig-4,5,6], MHD was significantly higher in left-sided breast cancer than right-sided (p-value <0.001 i.e., statistically highly significant, Mann-Whitney U Test). As depicted in [Table/Fig-7,8], the mean heart dose received during radiotherapy for left-sided breast cancer was found to be significantly higher than right-sided breast cancer (p-value <0.001 i.e., statistically highly significant, Independent t-test).
Mean, Standard error, standard deviation, Variance of Maximum Heart Distance (MHD), Mean Heart dose, V5 (Volume proportion of heart receiving 5Gy or higher dose), V20 (Volume proportion of heart receiving 20Gy or higher dose).
| Parmeters | N | Range | Minimum | Maximum | Mean | Standard error | Standard deviation | Variance |
|---|
| Mean heart dose (Gy) | 90 | 0-5.80 | 0 | 5.80 | 3.3687 | 0.19816 | 1.87994 | 3.534 |
| Heart- V5 | 90 | 0-18 | 0 | 18 | 9.7078 | 0.70021 | 6.64282 | 44.127 |
| Heart- V20 | 90 | 0-13 | 0 | 13 | 5.90 | 0.459 | 4.352 | 18.939 |
| Maximum heart distance (cm) | 90 | 0-4 | 0 | 4 | 1.99 | 0.157 | 1.489 | 2.217 |
Table showing mean and maximum values of mean heart dose and MHD in patients of carcinoma breast receiving radiotherapy. For right-sided breast cancer highest MHD was 0.5 cm and mean MHD was 0.017cm, maximum Mean heart dose was 1.53Gy while mean heart dose was 0.846Gy. For left-sided breast cancer, highest MHD was 4.3cm and mean MHD was 2.974cm, maximum mean heart dose was 5.80Gy while mean heart dose was 4.63Gy.
| Breast cancer | Average mean heart dose | Maximum mean heart dose | Mean maximum heart distance | Highest maximum heart distance |
|---|
| Right-sided breast cancer | 0.846 Gy | 1.5 Gy | 0.017 cm | 0.5 cm |
| Left-sided breast cancer | 4.63 Gy | 5.Gy | 2.974 cm | 4.3 cm |
Table showing mean and standard deviation of MHD in left and right-sided breast carcinoma patients. For right-sided breast cancer patients mean value of MHD was 0.017 with standard deviation 0.091. While for left-sided breast carcinoma, mean value of MHD was 2.974 with standard deviation 0.612 which was significantly higher than right-sided breast carcinoma patients.
| Maximum heart distance | Mean | Standard deviation | p-value |
|---|
| Right-sided breast carcinoma (n=30) | 0.017 | 0.091 | <0.001**(Mann-whitney U test) |
| Left-sided breast carcinoma (n=60) | 2.974 | 0.612 |
p<0.001 i.e., statistically highly significant
Comparison of mean Maximum Heart Distance in left-sided and right-sided breast cancer patients for those who have received radiotherapy. The Mean Maximum Heart Distance (MHD) for left-sided breast cancer was 2.974 cm which was significantly higher than right-sided breast cancer with mean value 0.017 cm (p-value 0.001** i.e., statistically highly significant, Mann-Whitney U-test).
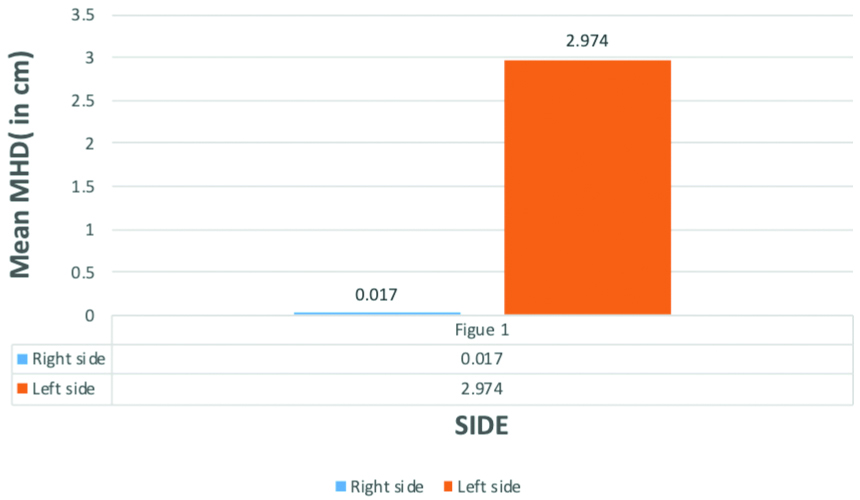
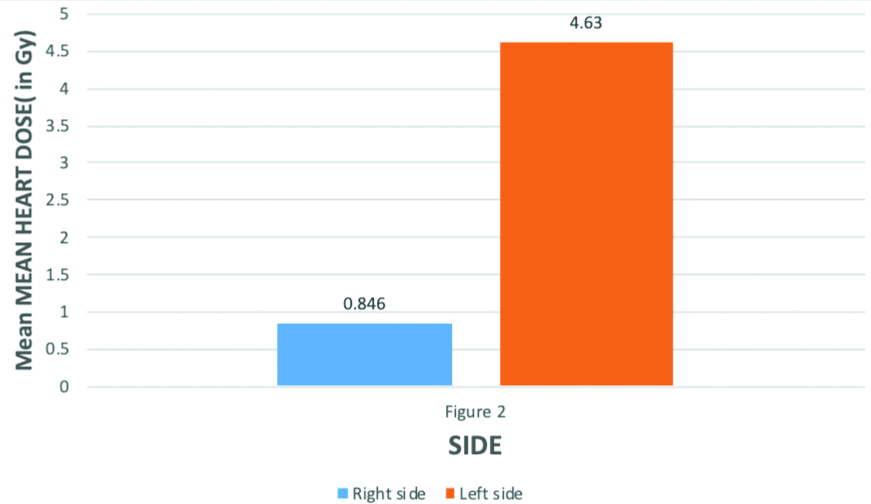
Table showing mean and standard deviation values of mean heart dose in left and right-sided breast carcinoma patients. For right-sided breast carcinoma mean value of mean heart dose was 0.8463 with standard deviation 0.311. For left-sided breast carcinoma mean value of mean heart dose was 4.629 with standard deviation 0.656 which was significantly higher than left sidedleft-sided breast cancer.
| Mean heart dose | Mean | Standard deviation | t-value | p-value |
|---|
| Right-sided breast carcinoma (n=30) | 0.8463 | 0.311 | 29.867 | 0.001** (Independent t-test) |
| Left-sided breast carcinoma (n=60) | 4.629 | 0.656 |
p<0.001 .e., statistically highly significant
Comparison of average of Mean Heart dose in left-sided and right-sided breast cancer for those who have received radiotherapy. The Mean Heart dose was significantly higher in left-side with mean value 4.63Gy than right side which have mean value 0.846Gy. p-value for difference is 0.001** i.e., statistically highly significant (independent t-test)
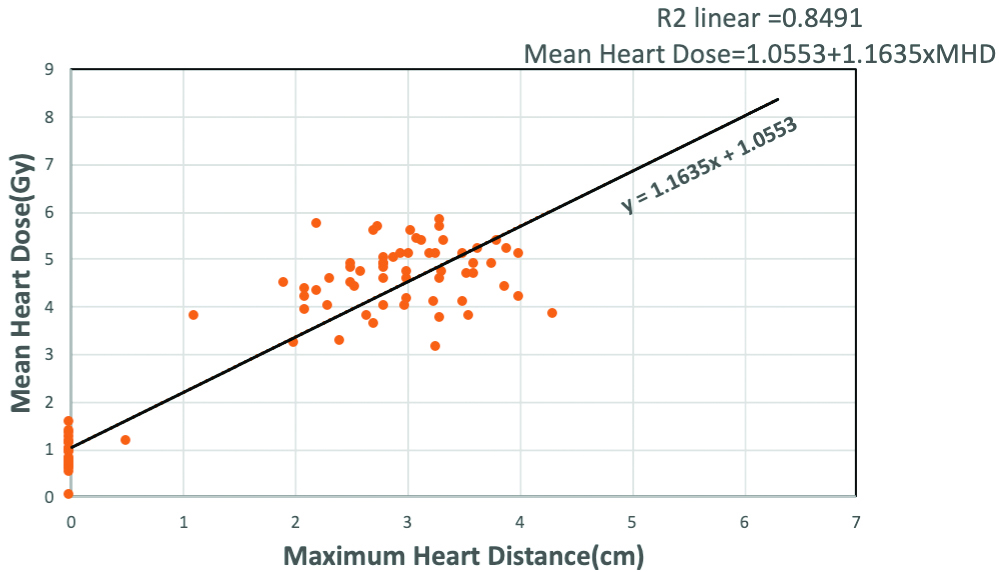
For all patients (both left-side and right-side breast cancer), there was a strong positive correlation between MHD and Mean Heart dose (p-value <0.001 i.e., statistically highly significant, Spearman Rho’s test). As shown in [Table/Fig-9], MHD and mean heart dose were compared by performing linear correlation analysis. The scattered plot showed strong linear positive correlation between MHD and Mean Heart Dose with Spearman Rho’s correlation coefficient R2=0.8491 with MHD on x-axis and Mean Heart Dose on y-axis and the relationship is given by the equation, Mean Heart Dose=1.0553+1.1635×MHD.
Scattered plot displaying relationship Mean heart Dose received with Maximum Heart Distance (MHD) in breast cancer patients receiving radiotherapy. MHD is denoted on x-axis and Mean Heart Dose denoted on y-axis. Mean Heart dose indicating a strong positive linear relationship with MHD with p-value 0.001** i.e., statistically highly significant. Relationship between MHD and Mean Heart Dose is shown as the trendline and given by the equation Mean Heart Dose=1.0553+1.1635×MHD, Spearman Rho’s correlation coefficient given by R2=0.8491.
Discussion
In this study, a comparison of dosimetrical indexes was established; all were thoroughly described in materials and methods section. The study was conducted to evaluate the individual dose to heart and correlation of MHD with Mean Heart Dose in Breast cancer radiotherapy. Long term cardiac toxicity of radiotherapy is a major concern for breast cancer patients and has been described in previous studies [4,6,9]. The cardiotoxicity due to radiation therapy leads to morbidity and mortality which is more in left-sided breast cancer as shown in various studies [3,4,6,9]. This is due to anatomical position of heart which leads to exposure of the heart to more dose of radiation in left-sided breast cancer than right-sided [4-7]. Darby SC et al., showed that there was no significant difference in mortality in left-sided breast cancer than right-sided breast cancer among those who were not irradiated, while mortality from heart disease increased significantly in left-sided breast cancer than right-sided, in those patients who received radiation [7]. Darby SC et al., found that the cardiac mortality ratio, left versus right tumour laterality, was 1.20 (CI 1.04-1.38, trend 2p=0.01) during the first decade after diagnosis, 1.42 (CI 1.11–1.82, 2p=0.005) during years 10-14, and 1.58 (CI 1.29-1.95, 2p=0·0001) after 15 or more years for women diagnosed during 1973-82 and irradiated [7]. Darby SC et al., further found that for women who were diagnosed with breast cancer during the time periods 1983-92 or 1993-2001, and who were subsequently treated with irradiation, the cardiac mortality ratios for left versus right tumour laterality, during only the first decade after diagnosis were 1.04 (CI 0.91-1.18) and 0.96 (CI 0.82–1.12), respectively [7].
The present study findings were in general accordance with other cardiotoxicity effect of radiotherapy studies [3,6,15,18] as mean heart dose was significantly higher in left-sided Ca breast (mean value 4.63Gy) than right-sided Ca breast (mean value 0.846Gy) with p-value <0.001. MHD is a simple radiographic parameter that measures the irradiated heart volume in breast cancer patient receiving radiotherapy [18]. Hurkmans CW et al., showed that Normal Tissue Complication Probability (NTCP) of cardiac mortality is 1% and 2% predicted at MHD of 1.2 cm and 1.6 cm, respectively [19].
MHD is also a well-founded predictor of Mean Heart Dose [20]. Taylor CW et al., found that there was a strong linear correlation between MHD and Mean Heart Dose where Mean Heart Dose=2.9 Gy/cm MHD+4.1Gy, R2=0.81 (R-multiple correlation coefficient) and average Mean heart dose was 5.1 GY [15]. Kong FM et al., found this relationship to be 2.8Gy/cm MHD+2.2 GY, R=0.76 [20]. Lorenzen EL et al., found it to be 2.4Gy/cm MHD+0.42Gy by performing linear regression analysis assuming a standard deviation of random and systemic error of 5 mm [21]. Mohamed W, in a retrospective analysis of 69 patients found that MHD significantly affected both mean heart dose and anterior descending coronary artery dose with Pearson correlation coefficient of 0.713 and 0.732, respectively [22]. Harder RJ also found that MHD had a strong linear correlation mean heart dose [23]. Aiello D et al., also found positive correlation between mean heart dose and MHD difference [24]. In this study, it was found that MHD was significantly in left-sided than right-sided breast cancer with p-value <0.001 and MHD was positively correlated with Mean heart dose. The relationship between MHD and Mean Heart Dose in breast cancer was found to be Mean Heart Dose=1.0553+1.1635×MHD, R2=0.8491 (R=Spearman Rho’s coefficient), found by performing linear correlation analysis. A meta-analysis Lai J et al., shows that, the DIBH group provided significantly lower doses to heart (SMD=-1.36, 95% CI -1.64 ~-1.09, p<0.01) and LADCA-left anterior descending coronary artery (SMD=-1.45, 95% CI -1.62~-1.27, p<0.01) than the free breathing group [25].
In accordance with this study, as dose to heart in right-sided breast cancer was negligible, a free breathing technique using three dimensional conformal radiotherapy will suffice. However, for patients with left-sided breast cancer requiring radiotherapy, cardiac sparing techniques like DIBH or ABC or IMRT scores over 3DCRT (3 dimensional conformal radiotherapy) [25].
Limitation(s)
Subgroup analysis of MRM and BCS was not done. Internal mammary chain irradiation group was not included in the study. Both right and left-sided breast cancer patients were treated with free breathing 3DCRT technique.
Conclusion(s)
The present study confirmed that Mean heart dose and MHD was higher significantly in left-sided breast cancer than right-sided breast cancer receiving radiotherapy. Therefore, cardiac tissue gets inadvertent irradiation more on the left-sided breast cancer. Also, MHD had strong positive correlation with Mean Heart Dose, so MHD too an independent predictor of cardiac dose. For right-sided breast cancer, free breathing technique using three-dimensional radiotherapy can be used, as it will expose heart to negligible dose of radiation.
p<0.001 i.e., statistically highly significant
p<0.001 .e., statistically highly significant