Incomplete, slow or limited wound healing is one of the major disabling events suffered by diabetic patients. The normal wound recovery becomes blocked at the inflammatory phase and revascularisation and tissue remodelling do not take place [1]. The condition can be sufficiently serious and can result in limb amputation. It is known that high glucose levels result in protein damage, whilst reducing circulating endothelial progenitor cells, which are essential to promote healing. Recently, lot of interest has been directed at the important properties of MSCs in disease and Xu J et al., recently showed that treatment of mouse diabetic wounds with MSCs reduced inflammation and aided recovery through a mechanism involving the microRNA miR-146a [2]. Some of the collaborators have demonstrated loading and slow release of drugs via MSC delivery that significantly benefited patients with cancer [3] and stem cell therapy which is now considered as one of the most exciting prospects to aid in the treatment of diabetic wound healing [4]. Since nano-silver wound dressings are already available for use and provide bactericidal properties, they form a perfect matrix for instigating combinational therapeutic approach [5]. Labelling of silver with the MSCs was carried out using published procedures [6] and with the assistance of nanotechnology company (Endor, Barcelona, Spain).
Recent developments in the fields of nanotechnology and stem cell research have enabled us to hypothesise that a combinational therapy employing the unique characteristics of a protective fibroin matrix, populated with MSCs loaded with drugs should produce significant beneficial effects over current therapeutic strategies [7]. As there are no effective treatments, which enable efficient wound healing in these patients, it was planned to employ the anti-inflammatory and anti-bacterial properties of fibroin matrix obtained from the pharmaceutical company Smith and Nephew (USA) and populate them with human MSCs loaded with SAC, a potent anti-glycating agent obtained from aged garlic extract [8]. This combination will allow slow release of the active substance into the wound over several days and together with the beneficial effects of the stem cells in promoting tissue remodelling, should result in significantly enhanced wound recovery [9]. The plan involved optimisation and preparation of the fibroin matrix and preliminary invivo testing using a mouse model of diabetes [10]. This could have significantly beneficial effects in the treatment of patients with diabetic complications associated with wound healing.
The objectives of this study were to culture and load MSCs with SAC from aged garlic extract and invitro optimisation of the delivery process, attachment and optimisation of stem cells and loaded stem cells to the fibroin matrix and comparison of fibroin dressing alone, dressing with stem cells and dressing with loaded stem cells in induction of wound recovery completion in a model of diabetic wound healing.
Materials and Methods
The study was approved by the Majmaah University Ethical Committee (BHSRC 28/6-T-11). Sheets of electro-spun silk fibroin (SF) [Table/Fig-1] were produced according to the method of Alessandrin A et al., and the stem cell loaded SF matrix and control patches were prepared according to method of Navone SE et al., [11,12].
Stem Cell Loading and Manipulations
Human Adipose Tissue-Derived Mesenchymal Stem Cells (Ad-MSCs) were obtained from Lonza and were cultured in Stem Cell Medium (SCM), consisting of DMEM/F12 (Dulbecco’s Modified Eagle Medium) supplemented with 10% Foetal Bovine Serum (FBS) (Gibco, Grand Island, NY, USA), 1% penicillin and streptomycin solution (Sigma-Aldrich, Basel, Switzerland) and optimised for the growth of stem cells. Stem cells were tested for: (1) ability to home and maintain themselves in a hypoxic environment and were modified to contain linker peptides; (2) uptake and delivery of NAC/SAC results to follow but the cells have taken up the drugs and we detected release into tissue culture medium-to be quantified by High Performance Liquid Chromatography (HPLC). We believe we can make the stem cells sensitive to pH and therefore, hypoxia and wound damage. The peptides showed no cytotoxic effects. Liposomes can also be employed in this system to assist in bio-delivery of the drugs.
Culturing MSC and Drug Loading and Monitoring
Preparation, culturing and loading of the MSCs was carried out according to the method Pessina A et al., [3]. Drug release into defined tissue culture medium was measured over time using an Enzyme-Linked Immunosorbent Assay (ELISA)-based assay. Attachment of MSCs to fibroin matrix was carried out using the method of Rehni AK et al., and kinetics, efficiency of labelling and release characteristics established and optimised invitro [6]. Monitoring effects on endothelial cells (EC) invitro was performed by EC and fibroblasts co-culture in normal and hypoxic conditions to represent ischemic cell damage and survival assays involving staining with antibodies to caspase-3 and administration of propidium iodide. Electron microscopy was used to identify detailed cellular structure and morphology as well as distribution of junctions. Briefly, cells (20,000) were allowed to adhere to micro wells (n=4). Peptide was added and incubated for 30 minutes. The wells were then, washed 3x with Phosphate-Buffered Saline (PBS) and buffer added, along with 10 μL of calcein liposomes. The plate was then, incubated for a further 30 minutes. Then, fluorescence intensity was read (Ex 485 nm Em 520 nm).
Characterisation of Protective Components for Anti-glycation/Oxidative Stress
Optimal components from aged garlic extract were isolated and tested for their protective effects against glycation and for promotion of vascular remodelling.
Measurement of the Effects of Fibroin Matrix, with and without MSCs, with and without Drug (SAC) Following Application to the Diabetic Mouse Model
To prepare the model, 20 Db/db diabetic JAX mice (The Jackson Labs) and their non-diabetic littermates were wounded. These mice were sorted into three groups and each group received a specific treatment, either with: (1) silver sheets (N=6); or (2) silver plus MSCs (N=7); or (3) silver plus MSCs with derivatives of aged garlic extracts (N=7). After each five days the wound was photographed by keeping the camera lens at a perpendicular axis till it was healed completely. These photographs were analysed using digital planimetric software [13] to find the efficacy of the used treatments. Wounds were then harvested, and relative expression of markers of inflammation and oxidative stress measured using an ABI Prism SDS 7000. Stem cell recruitment and incorporation was measured by flow cytometry and histology to assess the morphological appearance of the tissues.
Immunohistochemistry and Western Blotting were used to identify expression levels of wound healing proteins during the inflammatory and angiogenic phases and to identify the presence of specific cell types associated with correct and complete wound healing, contractile response.
Results
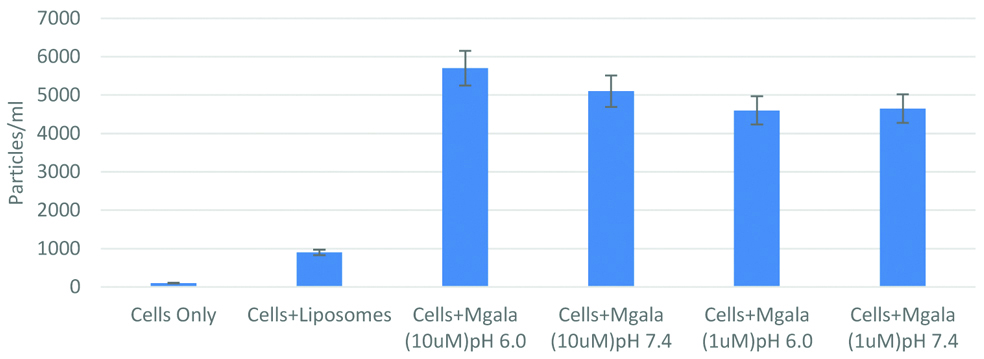
The data suggest that stem cells can be sensitised with a pore-forming peptide. This peptide can then, rupture liposomes while being associated with the stem cells. However, in this study it was found that the rupture of liposomes was not highly dependent on pH [Table/Fig-2]. It is encouraging to note that such stem cells may have the potential to be developed into bio-responsive delivery vehicles.
Characteristics of Mesenchymal Stem Cells (MSC) with nanocarriers like liposomes and silver nanoparticles (Mgala) at different pH and concentration displaying pH sensitive release of silver.
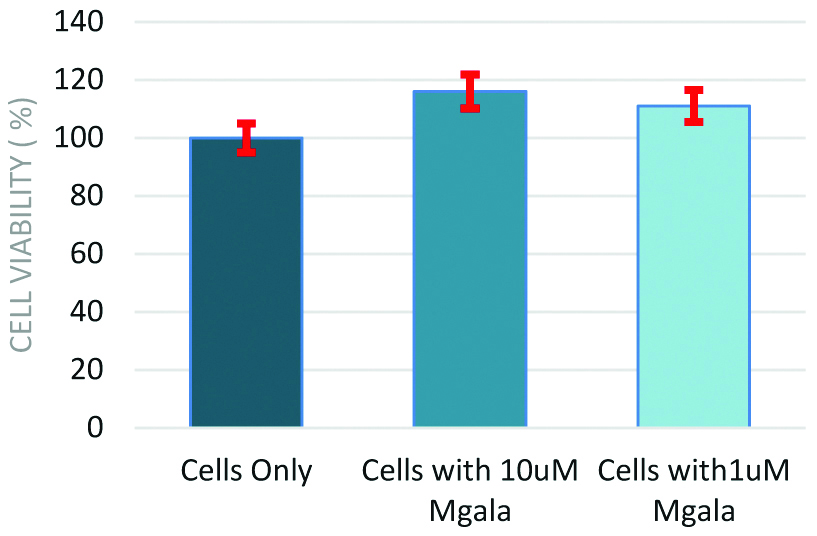
However, the risk usually is whether the cells remain viable once associated with the bio-responsive peptide. This was tested by a viability assay [Table/Fig-3]. The stem cells remain viable when associated with the pore-forming peptide. Taken all the data together, it may be possible to release drug payload from liposomes or drug loaded stem cells as a delivery vehicle.
Cell viability assay data of Mesenchymal Stem Cells (MSC) and silver nanoparticles (Mgala).
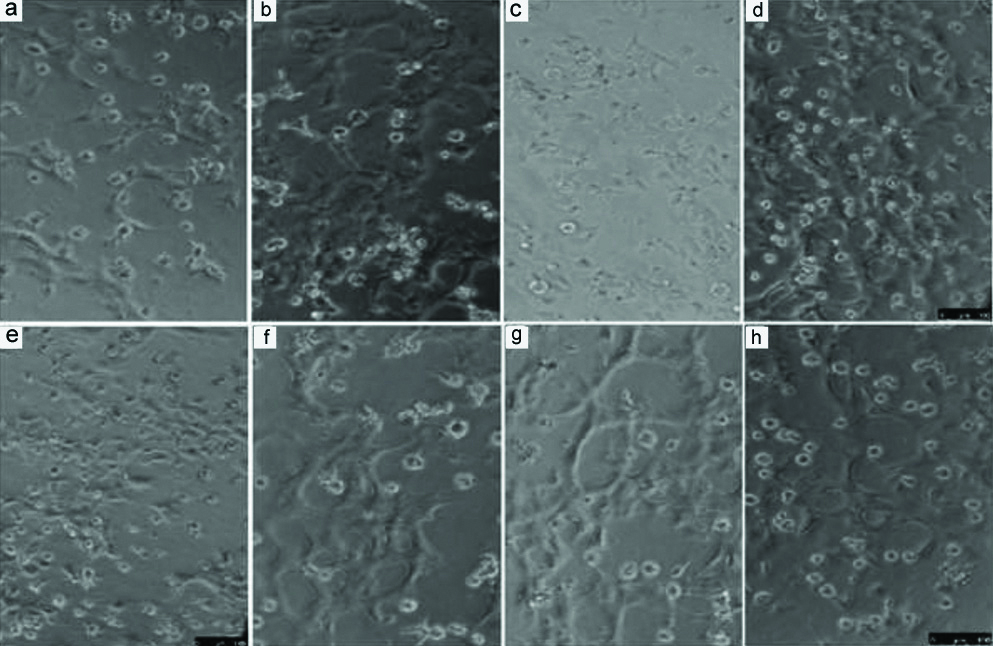
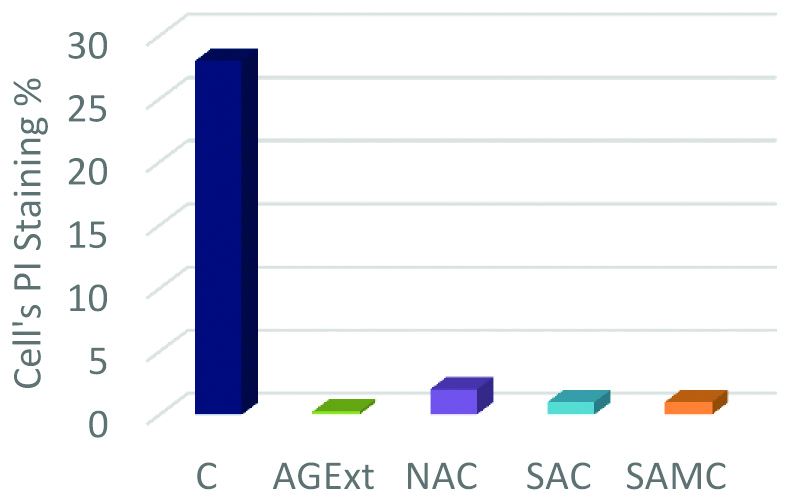
Mixture of SAC and NAC was most effective. SAMC protected angiogenesis as shown in the tube-formation assay on Matrigel- ‘F’ and ‘G’ shows tubes still forming in the presence of advanced glycation end-products [Table/Fig-4]. Protection against oxidative stress and apoptosis-PI staining shows protective effects of the cysteine compounds particularly SAC and SAMC [Table/Fig-5].
Tube formation assay on Matrigel. a) Control; b,c) Effect of N-acetylcysteine (NAC); d,e) Effect of SAC (S-allyl cysteine); f,g) Effect of S-allyl mercaptocysteine (SAMC) h) Effect of aged garlic extract (AgExt).
Propidium iodide (PI) staining to see the percentage of apoptotic cells and to see the protection against oxidative stress.
C: Control; AgExt: Aged garlic extract; NAC: N-acetylcysteine; SAC: S-allyl cysteine; SAMC: S-allyl mercaptocysteine
Discussion
Wound injuries in diabetic patients are notoriously difficult to heal with infections, burns, foot ulcers and deep cuts inevitably leading to hospitalisation and often, limb amputation [14]. Topical applications of a variety of substances have been used in attempts to improve diabetic wound healing, where the main problem seems to be a blockage of the normal remodelling pattern at the point of acute inflammation [15]. Excessive inflammation and oxidative stress pathway activation appear to block the normal progression towards re-vascularisation and tissue regeneration [16]. Some potentially useful agents such as genistein, which blocks inducible Nitric Oxide Synthase (iNOS) activity and reduces superoxide level have been shown to promote wound closure in diabetic rats [17]; the only drawback is their potential cytotoxic effects when used in humans. SAC has very strong anti-inflammatory effects and also inhibits oxidative stress [18]. Furthermore, it is safe to use in humans, being a component derived from aged garlic extract and therefore a natural food supplement.
Due to the beneficial effects in terms of cell attachment and bioactive factor loading, the tissue-engineered dermal tissue grafts have been successfully utilised for sealing deep skin wounds [19]. Han SK et al., in his study found no differences in cell proliferation and concluded that the stem cells produced much higher amounts of collagen and growth factors [20]. A study by An Y et al., demonstrated that MSCs improve cutaneous wound healing [21]. Another study showed the relationship between the mechanical loading of MSCs and improved vascularisation [22]. Saheli M et al., in a recent study established the usefulness of MSCs on fibroblast activity facilitating healing of chronic wound [23]. Fibroin spun matrix scaffolds effectively assist the dermal cells for accelerated dermal tissue formation by enabling a microenvironment exactly similar to Extracellular Matrix (ECM). In this regard, electro-spun fibres can improve the interaction between cells and scaffolds due to radically unremitting nanoscale fibres and variably porous composition simulating skin ECM resulting in enhanced attachment, proliferation, and differentiation of cells [24].
In recognition of the superiority of non-woven nanoscale fibrous scaffolds, electrospinning has emerged as a clear winner in terms of obtaining variously shaped structures for creating tissue-engineered skin grafts, which support the adhesion, spreading and proliferation of skin cells [25]. The cellularisation of these nanofibre matrices with drug-loaded MSCs make them an attractive choice for cell therapy as they augment the proliferative potential and produce a variety cytokines and growth factors important to wound healing, especially in diabetic patients.
Conclusion(s)
Nanofibre matrices support the adhesion, spreading and proliferation of skin cells whereas SAC with its strong anti-inflammatory and antioxidant activity preserves cell viability thereby, promoting wound healing. Present study results provide possibilities of utilising tissue-engineered fibroin matrix with drug-loaded human MSCs as a potential therapeutic technique, due to its beneficial effects in terms of cell attachment and bioactive factor loading, for diabetic wound healing.