Demyelinating lesions which present as solitary contrast-enhancing masses pose a diagnostic challenge for both radiologists and surgical neuropathologists and can mimic a number of intracranial space occupying lesions either neoplastic/inflammatory. Herein, a series of seven cases with particular emphasis on histopathological and immunohistochemical characterisation of these lesions was presented. The seven cases of Tumefactive Demyelinating Lesions (TDLs) diagnosed on histopathology include three males and four females with age ranging from 10 to 58 years (median-25 years) chiefly presenting with focal neurological deficits. Radiologically, the lesions were predominantly localised in the temporo-parietal region. Histologically, the lesions were well-demarcated, hypercellular with infiltration by foamy macrophages and reactive astrocytes. In addition, Creutzfeldt astrocytes and granular mitoses were seen. Additional special stains for myelin (Luxol fast blue), demonstrated focal, sharply marginated loss of myelin, and Immunohistochemistry (IHC) with Neurofilament (NF) for axons showed relative preservation of axons in areas of myelin loss. IHC with Glial Fibrillary Acidic Protein (GFAP) highlighted the fibrillary processes of the reactive astrocytes and the cytoplasm of the Creutzfeldt cells. Knowledge of the characteristic features such as well-demarcated lesions with histological characterisation by large number of macrophages, including Creutzfeldt astrocytes, granular mitoses and perivascular inflammation should raise index of suspicion and prompt pathologist to do additional myelin stains and IHC to confirm demyelination and preservation of intact axons thereby, differentiating from neoplasms and other non-neoplastic inflammatory lesions.
Creutzfeldt cells, Glioma, Mass effect, Perivascular lymphocytes, Tumefactive multiple sclerosis
Introduction
Multiple sclerosis (MS) is a mutifocal autoimmune inflammatory disorder of the CNS characterised by demyelination and progressive neurodegeneration. TDL is a variant of MS and mimics a number of intracranial space occupying lesions either neoplastic/inflammatory clinically and imageologically [1,2]. TDLs presenting as contrast-enhancing solitary lesions may pose a great diagnostic challenge to the clinician, radiologists and the surgical neuropathologists [2,3]. Many of these lesions respond to steroids, hence a high index of suspicion and early diagnosis is crucial to avoid aggressive treatment [4]. There are only very few case reports and few case series reported [5-8]. The series describe seven cases with particular emphasis on histopathological and immunohistochemical characterisation of these lesions.
Case Series
The demographic data, presenting clinical features and imageological data of seven cases histopathologically diagnosed as TDLs from January 2005 to December 2012 in the Department of Pathology, Nizams Institute of Medical Sciences, Hyderabad were obtained from medical records. The slides were reviewed with Haematoxylin and Eosin (H&E) along with special stain Luxol’ Fast Blue-Periodic Acid Schiff (LFB-PAS) stains. Immunohistochemistry (IHC) was performed with Glial Fibrillary Acidic Protein (GFAP) (Biogenex, California), NF (Biogenex, California) and CD68 (Biogenex, California). The morphological features evaluated include circumscription of the lesion from adjacent brain parenchyma, hypercellularity, axonal loss, demyelination, presence of macrophages, perivascular inflammatory cell aggregates, reactive gliosis, presence of gemistocytes, mitoses, areas of necrosis and haemorrhage.
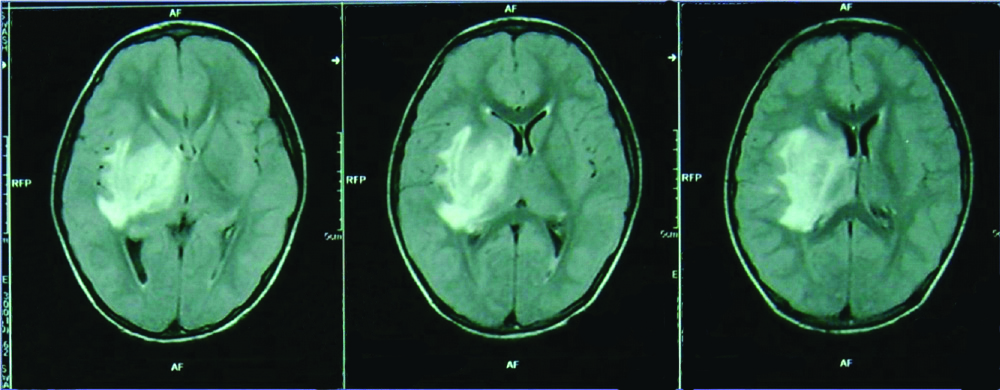
The seven cases during the study period include three males and four females with age ranging from 10 to 58 years (median-25 years). All the patients presented with focal neurological deficits. In addition two patients had headache, one had fever and aphasia [Table/Fig-1]. There was no history of remitting and relapsing illness or any immunosuppression. On MRI, the lesions were located in temporo-parietal region in three, parietal in two and multiple in two cases [Table/Fig-2].
Description of the cases.
| Serial No. | Age/Gender | Clinical features | Location in MRI | MRI features | Pre-operative diagnosis |
|---|
| 1. | 33/M | Weakness left upper limb and lower limb since three months | Right temporo-parietal | Suggestive of demyelination | Tumefactive demyelination |
| 2. | 25/F | HeadacheWeakness right upper limb since 20 days | Left parietal | Intracranial Space occupying Lesion with perilesional oedema | Glioma |
| 3. | 17/M | Fever, aphasiaWeakness left upper limb and lower limb | Multiple lesions | DemyelinationLymphoma | Lymphoma |
| 4. | 26/F | HeadacheWeakness left upper limb and lower limb | Right temporo-parietal | Heterogenous | Glioma |
| 5. | 10/M | Weakness right upper limb and lower limb | Multiple | Suggestive ofGliomaTumefactivedemyelination | ?Tumefactive demyelination? Glioma |
| 6. | 58/F | Weakness left lower limb since two months | Right temporo-parietal | Suggestive of right temporo-parietal lesion | Glioma |
| 7. | 26/F | Weakness right upper limb and lower limb since one month | Left parietal | Suggestive of left parietal lesion | ?Glioma |
MRI: Magnetic resonance imaging
MRI image showing hyperintense lesion involving the right capsuloganglionic (Case 7).
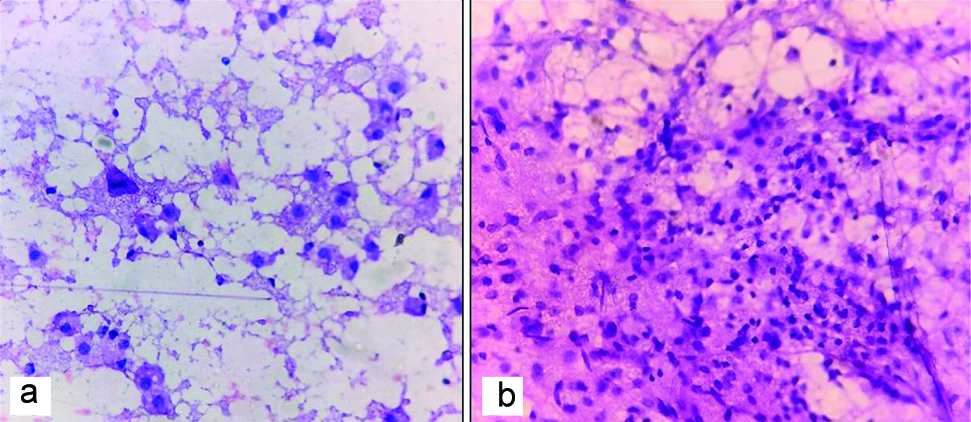
Intraoperative smears were available in three cases. The smears showed macrophages with variable number of reactive astrocytes [Table/Fig-3]. Few perivascular lymphocytes were seen in one case. Diagnosis of reactive gliosis and infarct were made in one case each. In the case where perivascular lymphocytes were seen, a possibility of demyelination was suggested.
Rapid H and E smears of squash images showing a) Numerous foamy macrophages (200 x): b) Lymphocytic collection; (200x).
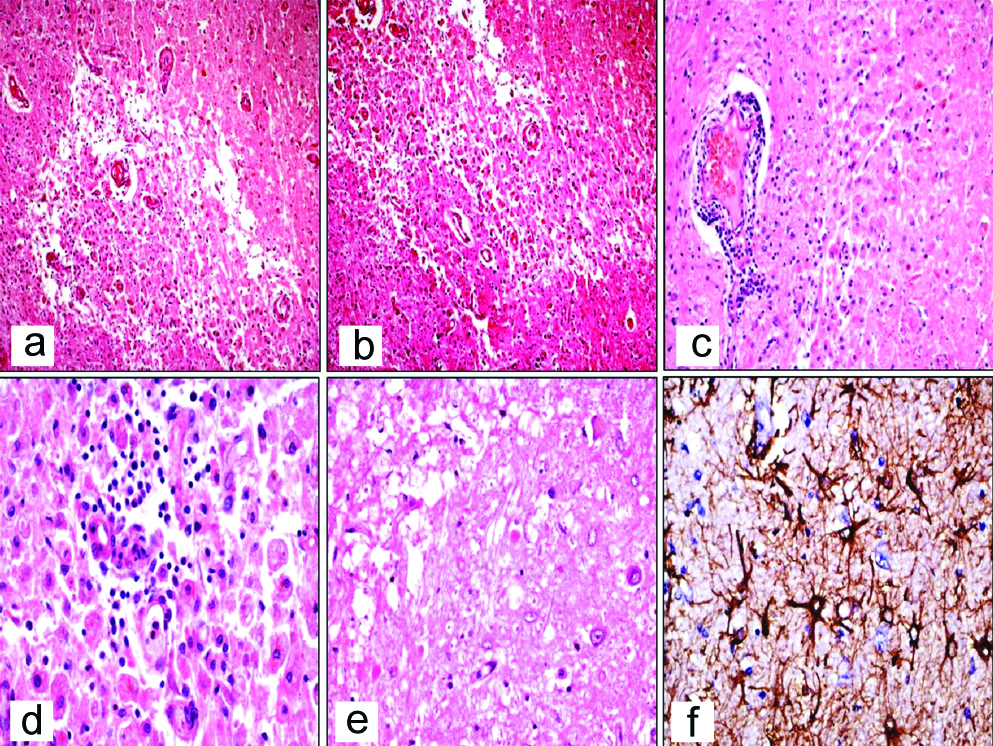
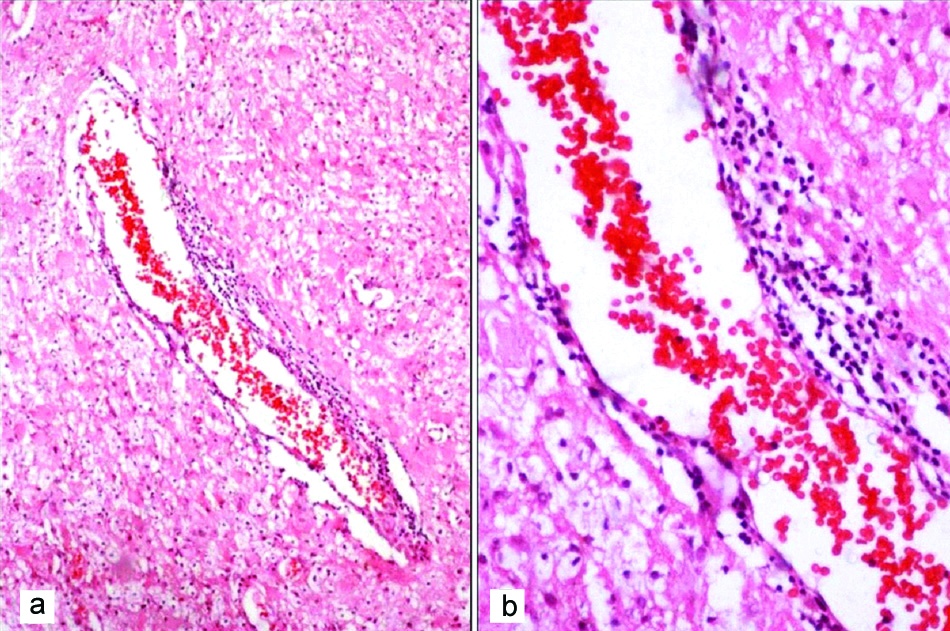
On histology, the lesions were typically demarcated from adjacent brain parenchyma. The lesions were hypercellular with infiltration by foamy macrophages [Table/Fig-4a,b] along with perivascular lymphocytic infiltration seen in all cases [Table/Fig-4c,d]. Amidst, the macrophages were evenly distributed reactive astrocytes with eosinophilic cytoplasm and multipolar processes [Table/Fig-4e]. IHC with GFAP highlighted the fibrillary processes of the reactive astrocytes and the cytoplasm of the Creutzfeldt cell [Table/Fig-4f]. The macrophages had distinct cell borders, foamy/pale granular cytoplasm and small dense nuclei [Table/Fig-5]. There were large astrocytic cells with abundant glassy cytoplasm and multiple micronuclei (Creutzfeldt cells) in four biopsies and granular mitoses were also observed in four biopsies [Table/Fig-6]. LFB-PAS stain showed sharply defined area of demyelination and some PAS positive granular material within the macrophages [Table/Fig-7]. IHC with Cluster of Differentiation (CD68) delineated the macrophages and the margin of lesion. The preserved axons were highlighted by IHC with NF [Table/Fig-8].
(a,b) Hypercellular lesion with infiltration by foamy macrophages (H and E, 40x); (c,d) Distinct perivascular inflammatory infiltrate (H&E, 40x and 400x); e) Reactive Astrocytes with eosinophilic cytoplasm (H&E, 100 x); f) Reactive Astrocytes show intense GFAP positivity (IHC,100x).
Case 5 showing collection of foamy macrophages with abundant glassy cytoplasm with perivascular lymphocytic collection (H&E) a) 200x, b) 400x.
a) Large astrocytic cells with abundant glassy cytoplasm and multiple micronuclei (Creutzfeldt cells); b) Granular mitosis; (H&E, 400x).
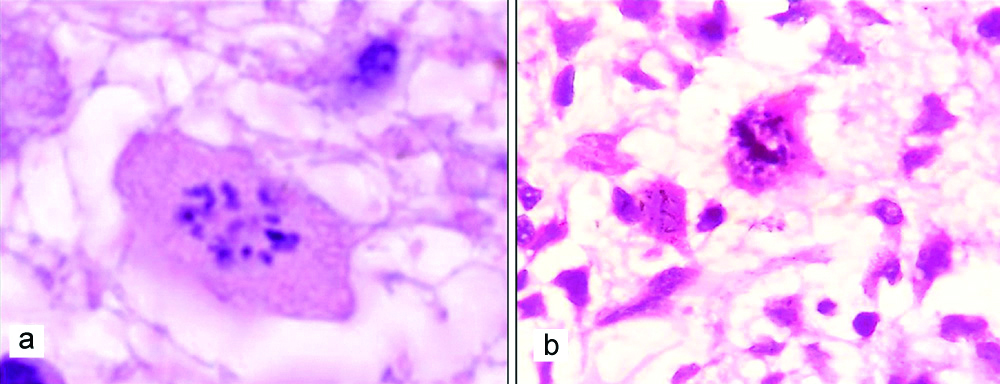
a) LFB-PAS stain showing loss of myelin with distinct boundaries from adjacent myelinated white matter (LFB-PAS stain, 100 x); b) Accumulation of degraded myelin within histiocytes (LFB-PAS stain, 400x).
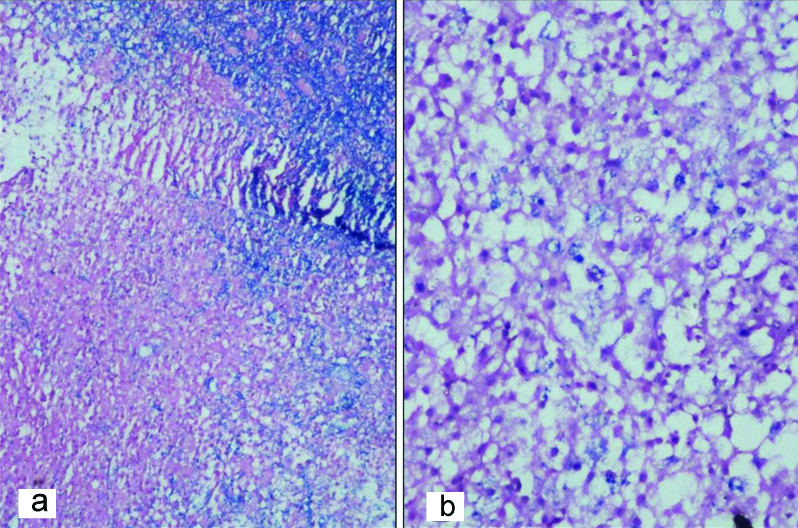
a) Positivity for CD68 in histiocytes (IHC,100 x); b) Intact axons shown by neurofilament immunostain (IHC, 400 x).
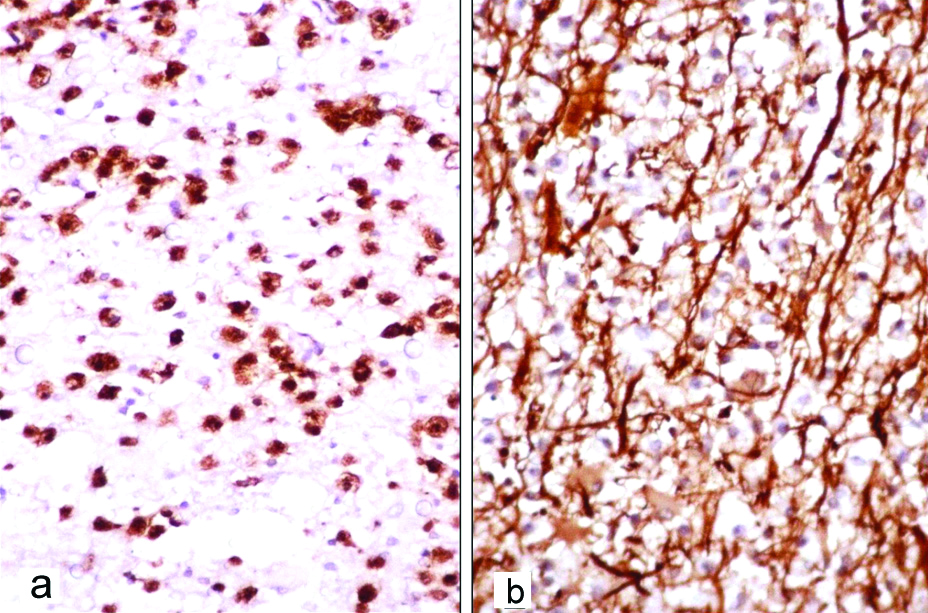
The provisional differential diagnoses with various histopathological and immunohistochemical findings are summarised in [Table/Fig-9].
Summary of histopathological features with special stains and IHC results.
| S. No. | Squash findings | Squash Dx | Histoapthology findings | LFB-PAS | CD 68 | NF | GF-AP In RA | Final Dx |
|---|
| FM | PLI | RA and CZ cells | Granular mitosis | Provisional Dx |
|---|
| Case 1 | Not done | - | + | + | - | - | InflammatoryInfarctTDL | DM+ | + | Preservation of axons | Not done | CNS Demyelination |
| Case 2 | Reactive astrocytes | Reactive gliosis | + | + | + | + | GliomaLymphomaTDL | DM+ | + | Preservation of axons | + | Demyelinating Pseudotumour |
| Case 3 | Not done | - | + | + | - | - | InflammatoryInfarctTDL | DM+ | + | Preservation of axons | Not done | Demyelinating Pseudotumour |
| Case 4 | Not done | - | + | + | + | + | GliomaTDL | DM+ | + | Preservation of axons | + | Tumefactive demyelinatinglesion |
| Case 5 | Perivascular lymphocytes with FM | TDL | + | + | + | + | GliomaTDL | DM+ | + | Preservation of axons | + | Demyelinating Pseudotumour |
| Case 6 | Reactive astrocytes | Infarct | + | + | + | + | GliomaTDL | DM+ | + | Preservation of axons | + | Demyelinating Pseudotumour |
| Case 7 | Not done | - | + | + | - | - | InflammatoryInfarctTDL | DM+ | + | Preservation of axons | Not done | Demyelinating Pseudotumour |
FM: Foamy macrophages; PLI: Perivascular lymphocytes; RA: Reactive astrocytes; CZ: Cruetzfeldt cells; LFB-PAS: Luxol fast blue-Periodic acid schiff stain; NF: Neurofilament; GFAP: Glial fibrillary acidic Protein; DM: Demyeliantion; TDL: Tumefactive demyelination; CNS: Central nervous system; Dx: Diagnosis; (+)- present, (-) absent
Discussion
Acute demyelinating disorders such as MS and Acute Demyelinating Encephalomyelitis (ADEM) presenting as TDL poses diagnostic problem as they closely mimick primary brain tumours. Subset of patients present typically with large solitary white matter lesions greater than 2 cm with perilesional oedema associated with mass effect and contrast enhancement masquerading an intracranial neoplasm. The prevalence of TDL is estimated to be 1-3/1000 with incidence estimated at 0.3 cases per 100000 population [9]. They may occur in isolation or more commonly in established MS. Atypical clinical or radiological manifestation in an established MS can suggest concurrent neoplams owing to similar clinical presentations [10,11]. These lesions typically showing perilesional oedema with ring enhancement in MR imaging which clinically and radiologically may mimic primary and secondary brain tumour, brain abscess, tuberculous abscess, or other inflammatory disorders.
The usual clinical manifestations of TDL are related to mass effect and are variable, depending on lesion location and size, and include headache, cognitive changes, mental confusion, aphasia and apraxia, neurological deficits, seizures [12,13]. All the patients presented with monophasic illness and had no association with previous diagnosis of MS and presented with focal neurological deficits and few cases along with headache and aphasia. TDLs on MRI show large white matter lesiona with ill-defined borders, perilesional vasogenic oedema, relatively low cerebral blood volume, central necrosis, cystic change, incomplete or open ring contrast enhancement, variable involvement of grey matter and vessel-like structures travesing through the center of the lesion [14], hence clinically and radiologically may mimic primary and secondary brain tumour, brain abscess, tuberculous abscess, or other inflammatory disorders. Certain features on MRI like open ring enhancement, peripheral restriction on diffusion weighted imaging, vascular enhancement were suggested as useful parameters for differentiating TDLs from tumour [3,5]. In this series, diagnoses of TDL was suspected in two cases. Glioma was the pre-operative diagnosis in majority (5/7) of the cases. A possibility of lymphoma was considered in one case. In most of the reported series, the preoperative diagnosis was glioma [4,6]. The lesions in this series were solitary in five and multiple in two cases. Elevation of glutamate/glutamine peaks on Magnetic Resonance Spectroscopy (MRS) can be helpful in differentiating TDL from tumours [5,15].
Patients with clinically monophasic solitary lesion or atypical ring enhancing lesions and mass effect may require biopsy to establish diagnosis [13,15,16]. The diagnosis on TDL on pre-operative squash smear can be difficult. The main differential diagnoses includes infarct, inflammatory lesions, xanthomatous lesions and CNS lymphomas treated with corticosteroids [17]. Diagnosis was suggested in only one out of three cases in this series. Hence, a clinicoradiological correlation is necessary and pathologist at best can confirm abnormal tissue in smears.
Histolopathological findings of a sharply demarcated white matter lesion with macrophage infiltration, reactive astrocytes and perivascular lymphocytic aggregates point towards a non-neoplastic pathology and should prompt the pathologist to do additional stains for myelin and IHC for axons and CD68. The preservation of intact axons together with demyelination confirms the diagnosis of TDLs. The distribution of macrophages and reactive astrocytes also gives a clue to the diagnosis [7,15]. The lymphocytic infiltrate in the perivascular area varies with the activity of the disease and the perivascular inflammation can be seen within and outside the lesional area [7]. The aggregates of macrophages, the bizzare nuclei of the reactive astrocytes and granular mitosis lead to a mistaken diagnosis of glioma especially oligodendroglioma and astrocytoma. Foamy macrophages can occur in a few gliomas like pleomorphic xanthoastrocytoma, lipidized glioblastoma and others but the age, location, imageology and pattern of distribution of macrophages within the lesion along with demyelination and IHC with CD68 help in the differential diagnosis [7,8]. The presence of macrophages and reactive astrocytes also raises the possibility of infarct but the location and preservation of intact axons help in making the diagnosis. Both axons and myelin are lost in infarct. Infarct usually involves the cortex and hence, may show red neurons (hypoxic) and thick capillaries. Progressive Multifocal Leukoencephalopathy (PML) can also show presence of macrophages with areas of demyelination but a prior history of immunosuppression and presence of ground glass inclusions in oligodendroglial nuclei which can be confirmed on IHC, differentiates it from TDL [8]. Multiple lesions, treated with corticosteroids with a diagnosis of lymphoma have large number of macrophages posing problems on histology. The enhancing pattern on imageology favour a lymphoma and presence of demyelination and younger age favour TDL.
Conclusion(s)
A detailed neurological history, examination and radiological findings are essentially important in dealing with patients presenting as space occupying single/multiple white matter lesions. The particular attention to prior episodes of transient neurological dysfunction for which the patient may not have sought medical attention may be very helpful specifying features more characteristic to demyelinating disease. There are specific histopathological findings which can be helpful in arriving at a conclusive diagnosis, provided there is a high index of suspicion to have to consider a demyelinating lesion leading to in-addition routine histopathological protocols for special staining for myelin and axons.
MRI: Magnetic resonance imaging
FM: Foamy macrophages; PLI: Perivascular lymphocytes; RA: Reactive astrocytes; CZ: Cruetzfeldt cells; LFB-PAS: Luxol fast blue-Periodic acid schiff stain; NF: Neurofilament; GFAP: Glial fibrillary acidic Protein; DM: Demyeliantion; TDL: Tumefactive demyelination; CNS: Central nervous system; Dx: Diagnosis; (+)- present, (-) absent
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jun 08, 2020
Manual Googling: Sep 18, 2020
iThenticate Software: Oct 27, 2020 (6%)
[1]. Ning X, Zhao C, Wang C, Zhang D, Luo Q, Intracranial demyelinating pseudotumour: A case report and review of the literatureTurk Neurosurg 2017 27(1):146-50. [Google Scholar]
[2]. George T, Cicilet S, Hoisala R, Rout P, Multifocal tumefactive demyelination mimicking intracranial neoplasmJ Clin Diagn Res 2016 10(3):TD10-11.10.7860/JCDR/2016/15589.746527134967 [Google Scholar] [CrossRef] [PubMed]
[3]. Malhotra HS, Jain KK, Agarwal A, Singh MK, Yadav SK, Hussain M, Characterisation of tumefactive demyelinating lesions using MR imaging and in-vivo proton MR spectroscopyMult Scler 2009 15:193-203.10.1177/13524585080979221918177 [Google Scholar] [CrossRef] [PubMed]
[4]. Höftberger R, Guo Y, Flanagan EP, Sebastian Lopez-Chiriboga A, Endmayr V, Hochmeister S, The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibodyActa Neuropathol 2020 139(5):875-92.10.1007/s00401-020-02132-y32048003 [Google Scholar] [CrossRef] [PubMed]
[5]. Sinha MK, Garg RK, Bhatt M, Chandra A, Tumefactive demyelinating lesion: Experience with two unusual patientsJ Postgrad Med 2010 56:146-49.10.4103/0022-3859.6529220622396 [Google Scholar] [CrossRef] [PubMed]
[6]. Nadkar MY, Deore RA, Singh R, Tumefactive demyelinationJ of the Associ of Physici of India 2008 56:901-03. [Google Scholar]
[7]. Jain D, Rajesh LS, Vashista RK, Radotra BD, Banerjee AK, Demyelinating disease simulating brain tumours: A histopathologic assessment of seven casesIndian J Med Sci 2006 60:47-52.10.4103/0019-5359.1991216505573 [Google Scholar] [CrossRef] [PubMed]
[8]. Neelima R, Krishnakumar K, Nair MD, Kesavadas C, Hingwala DR, Radhakrishnan VV, Tumefactive demyelinating lesions: A clinicopathological correlative studyIndian J Pathol Microbiol 2012 55(4):496-500.10.4103/0377-4929.10778823455787 [Google Scholar] [CrossRef] [PubMed]
[9]. Ragel BT, Fassett DR, Baringer JR, Browd SR, Dailey AT, Decompressive hemicraniectomy for tumefactive demyelination with transtentorial herniation: ObservationSurg Neurol 2006 65:582-83.10.1016/j.surneu.2005.08.01516720180 [Google Scholar] [CrossRef] [PubMed]
[10]. Given CA, Stevens BS, Lee C, The MRI appearance of tumefactive demyelinating lesionsAJR Am J Roentgenol 2004 182:195-99.10.2214/ajr.182.1.182019514684539 [Google Scholar] [CrossRef] [PubMed]
[11]. Fallah A, Banglawala S, Ebrahim S, Paulseth JE, Jha NK, Case series: Tumefactive demyelinating lesions: A diagnostic challengeCan J Surg 2010 53:69-70. [Google Scholar]
[12]. Masdeu JC, Quinto C, Olivera C, Tenner M, Leslie D, Visintainer P, Open-ring imaging sign: Highly specific for atypical brain demyelinationNeurology 2000 54:1427-33.10.1212/WNL.54.7.142710751251 [Google Scholar] [CrossRef] [PubMed]
[13]. Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosisBrain 2008 131:1759-75.10.1093/brain/awn09818535080 [Google Scholar] [CrossRef] [PubMed]
[14]. Saindane AM, Cha S, Law M, Xue X, Knopp EA, Zagzag D, Proton MR spectroscopy of tumefactive demyelinating lesionsAJNR Am J Neuroradiol 2002 23:1378-86. [Google Scholar]
[16]. Cianfoni A, Niku S, Imbesi SG, Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopyAJNR Am J Neuroradiol 2007 28:272-77.10.1016/S0098-1672(08)70230-3 [Google Scholar] [CrossRef]
[17]. Tan HM, Chan LL, Chuah KL, Goh NS, Tang KK, Monophasic, solitary tumefactive demyelinating lesion: Neuroimaging features and neuropathological diagnosisBr J Radiol 2004 77:153-56.10.1259/bjr/2668260715010391 [Google Scholar] [CrossRef] [PubMed]
[18]. Schwartz KM, Erickson BJ, Lucchinetti C, Pattern of T2 hypointensity associated with ring-enhancing brain lesions can help to differentiate pathologyNeuroradiology 2006 48:143-49.10.1007/s00234-005-0024-516447037 [Google Scholar] [CrossRef] [PubMed]