Oral Squamous Cell Carcinoma (OSCC) is the commonest malignant neoplasm of the oral cavity. It is a widely occurring cancer and its incidence is highest in India, South and Southeast Asian countries [1]. OSCC’s incidence correlates with the intensity and longevity of carcinogenic exposure i.e., tobacco and alcohol consumption [2]. Thus, the cases predominantly occur in males over fifty years of age and are rare in young patients [2]. The incidence of OSCC in subjects less than 40 years ranges from 0.4 to 3.9% [2,3]. However, the relative incidence seems to be increasing in younger patients worldwide [2-4]. Studies conducted in Asia, Africa and Middle-East has shown the highest frequencies of OSCC in young patients [4]. Unlike older patients, there is no consensus regarding the clinicopathological characteristics, biological behaviour and prognosis of OSCC in the younger patients [2]. Further the aetiopathogenesis, including the role of genetic susceptibility, environmental influences, immune status and viral infections; have not been clearly elucidated in the young subjects [3]. Some studies concluded that OSCC in young exhibits poorer prognosis and aggressive behaviour when compared to their older counterparts, whereas others suggested a similar clinical outcome in both the groups [2,3]. Thus, the objectives of this study were to evaluate the clinicopathological features of OSCC in younger patients and to determine any characteristic specific to this population in contrast to the older subjects.
Materials and Methods
This single centre prospective study was conducted on radical resection specimens of oral cavity carcinomas, received in the Department of Pathology, for routine histopathological evaluation from the Department of Surgical Oncology, MS Ramaiah Medical College and Hospitals, Bengaluru, over a period of 5 years (between April 2015 and April 2020), after obtaining the Institutional Ethical clearance and patients informed consent. Sample size was calculated based on a previous study by Sheoni R et al., in which the percentage of OSCC was 27% in patients <40 years of age. In the present study, considering the expected proportion as 27%, precision of 7% and 95% confidence level, the minimum overall sample size (i.e., including the young and old patients) was estimated to be 155 [5]. However, we have included 187 samples. Cases with recurrent OSCC, metastatic squamous cell carcinoma and salivary gland carcinomas and excision specimens without lymph node resection were excluded. After a detailed gross specimen examination (including tumour site, appearance and size), multiple tissue bits were taken from the tumour, surgical margins and all the lymph nodes. The latter were processed as per standard protocol and paraffin embedded blocks were cut and stained by Haematoxylin and Eosin (H&E). The H&E stained slides were studied for the tumour histology, grade, perineural and lymphovascular invasion, WOPI, lymph node metastasis and extranodal extension and other features. The tumours were staged according to “Pathologic TNM Staging of Lip and Oral cavity, American Joint Committee on Cancer (8th edition-2017) staging system” [6]. OSCC were distributed according to primary sites as per AJCC 8th ed. i.e., mucosal lip (C00), buccal mucosa (C06.0), lower alveolar ridge (C03.1), upper alveolar ridge (C03.0), retromolar trigone (C06.2), floor of mouth (C04), hard palate (C05) and tongue (C02) [6]. Clinical details including risky habits (tobacco usage and alcohol consumption) and family history of OSCC were obtained from patient’s case files.
The clinicopathological characteristics in young patients (≤40 years) were compared to those of older patients (>40 years). However, we have included 187 samples.
Statistical Analysis
SPSS Version 18.0 software was used for analysis. All the categorical variables were expressed as frequency and percentage. The categorical variables were further analysed using the chi-square test to determine whether there were significant differences in the factors like risky behaviour, tumour morphology etc., between the study groups (≤40 years and >40 years). The p-value <0.05 was considered as statistically significant.
Results
There were 187 patients with OSSC over the past 5 years, comprised of 35 (18.7%) young patients and 152 (81.3%) older patients. The mean age was 36.8 years in young patients (28 to 40 years) and 59.9 years in older patients (41 to 83 years). The male: female ratio was 1.3:1 and 1.9:1 in the young and older patients respectively. Majority of the cases occurred in the 7th decade (27.3%, 51/187) followed by 6th decade (21.9%, 41/187) and in both the study groups males predominated {57.1% (20/35) in young and 65.8% (100/152) in older patients} [Table/Fig-1]. The youngest patient was 28-year-old without any adverse habits and oldest patient was 83-year-old with tobacco chewing habit.
Age and sex distribution.
| Age group (years) | No. of cases (%) | Mean age (years)±SD | Male (%) | Female (%) | Male:Female ratio |
|---|
| 20-30 | 4 (2.1) | 29.5±0.82 | 3 | 1 | 3:1 |
| 31-40 | 31 (16.6) | 37.8±2.48 | 17 | 14 | 1.2:1 |
| 41-50 | 35 (18.7) | 45.9±2.82 | 24 | 11 | 2.2:1 |
| 51-60 | 41 (21.9) | 56.3±3.06 | 28 | 13 | 2.2:1 |
| 61-70 | 51 (27.3) | 65.3±2.89 | 32 | 19 | 1.7:1 |
| 71-80 | 22 (11.8) | 74.1±1.96 | 13 | 9 | 1.4:1 |
| 81-90 | 3 (1.6) | 81.7±0.94 | 3 | 0 | - |
| Total | 187 (100) | 55.7±13.03 | 120 (64) | 67 (36) | 1.8:1 |
Risk factors of OSCC were present in 89.3% (167/187) of the patients {85.7% (30/35) in young and 90.1% (137/152) in older patients} [Table/Fig-2,3]. A 58.8% (110/187) of the patients were tobacco users, either in the form of chewing, smoking or both. A 7.5% (14/187) of the patients had history of only alcohol consumption. A 22.9% (43/187) of patients had habit of both tobacco and alcohol consumption [Table/Fig-2]. However, in view of insufficient data, it was not possible to analyse these risk habits in terms of quantity, duration of exposure and frequency of abuse.
Risk factor exposure among the age groups.
| Risk factors | Young patients (≤40 years) n=35 (%) | Older patients (>40 years) n=152 (%) |
|---|
| Nil | 5 (14.3) | 15 (9.9) |
| Adverse habits | Tobacco | 19 (54.3) | 91 (59.8) |
| Alcohol | 4 (11.4) | 10 (6.6) |
| Alcohol+Tobacco | 7 (20) | 36 (23.7) |
Comparison of sex distribution, habits and tumour macroscopy between the two groups; OSCC: Oral squamous cell carcinoma; p-value <0.05 to be considered significant
| Parameter | Young patients (≤40 years) n=35 (%) | Older patients (>40 years) n=152 (%) | p-value |
|---|
| Sex | Male | 20 (57.1) | 100 (65.8) | 0.336 |
| Female | 15 (42.9) | 52 (34.2) |
| Risky habits | Nil | 5 (14.3) | 15 (9.9) | 0.984 |
| Present | 30 (85.7) | 137 (90.1) |
| Family history of OSCC | 2 (5.7) | 1 (0.7) | 0.031 |
| Tumour site | Lip | 0 | 2 (1.3) | 0.58 |
| Buccal Mucosa | 19 (54.3) | 79 (52) |
| Lower alveolar ridge | 6 (17.1) | 26 (17.1) |
| Upper alveolus ridge | 0 | 3 (2) |
| Retromolar trigone | 3 (8.6) | 15 (9.9) |
| Floor of mouth | 0 | 2 (1.3) |
| Hard palate | 0 | 4 (2.6) |
| Tongue | 7 (20) | 21 (13.8) |
| Tumour Morphology | Exophytic | 21 (60) | 92 (60.5) | 0.954 |
| Endophytic | 14 (40) | 60 (39.5) |
In the young, majority of the cancers occurred in the buccal mucosa (54.3%, 19/35) followed by tongue (20%, 7/35) whereas buccal mucosa (52%, 79/152) and lower alveolar ridge (17.1%, 26/152) were the major sites in the older patients. In both the groups, exophytic morphology was the commonest presentation [Table/Fig-3].
As depicted in [Table/Fig-4], majority of the tumours were moderately differentiated {62.9% (22/35) in young and 66.4% (101/152) in older patients}. Extranodal extension, lymphovascular invasion and WOPI were relatively frequent in the young compared to older patients [Table/Fig-4].
Microscopic characteristics of OSCC among the two groups.
| Characteristics | Young patients (≤40 years) n=35 (%) | Older patients (>40 years) n=152 (%) | p-value |
|---|
| Tumour type and differentiation | OSCC, well differentiated | 10 (28.6) | 41 (27) | 0.62 |
| OSCC, moderately differentiated | 22 (62.9) | 101 (66.4) |
| OSCC, poorly differentiated | 1 (2.9) | 7 (4.6) |
| Verrucous carcinoma | 2 (5.7) | 3 (2) |
| Perineural invasion | 3 (8.6) | 14 (9.2) | 0.905 |
| Extranodal extension | 5 (14.2) | 14 (9.2) | 0.370 |
| Lymphovascular invasion | 14 (40) | 42 (27.6) | 0.301 |
| Worst pattern of invasion (WOPI-5) | 3 (8.6) | 10 (6.5) | 0.676 |
OSCC: Oral squamous cell carcinoma; p-value <0.05 to be considered significant
As depicted in [Table/Fig-5], young patients presented at high T stage (stages T3 and T4) (57.1%, 20/35) compared to older patients (46.7%, 71/152), and had higher frequency of lymph node metastasis {40% (14/35) vs. 25.6%(39/152)}. Higher proportion of young patients presented at advanced stage (stages III and IV) compared to older patients {77.2% (27/35) vs. 68.4% (104/152)} [Table/Fig-5].
Comparison of TNM classification among the two groups.
| TNM classification | Young patients (≤40 years) n=35 (%) | Older patients (>40 years) n=152 (%) | p-value |
|---|
| T stage | T1 | 5 (14.3) | 16 (10.5) | 0.485 |
| T2 | 10 (28.6) | 65 (42.8) |
| T3 | 13 (37.1) | 45 (29.6) |
| T4 | 7 (20) | 26 (17.1) |
| N stage | N0 | 21 (60) | 113 (74.3) | 0.323 |
| N1 | 7 (20) | 16 (10.5) |
| N2 | 5 (14.3) | 18 (11.8) |
| N3 | 2 (5.7) | 5 (3.3) |
| TNM stage | I | 1 (2.9) | 9 (5.9) | 0.751 |
| II | 7 (20) | 39 (25.7) |
| III | 15 (42.9) | 56 (36.8) |
| IV | 12 (34.3) | 48 (31.6) |
p-value as determined by chi-square test; TNM: Tumor node metastasis; p-value <0.05 to be considered significant
Discussion
An OSCC occurs predominantly in the older patients with peak age frequencies in the fifth to seventh decades of life [7,8]. However, literature review reveals an increasing trend in the younger population with frequencies ranging from 4 to 28% [2,3,7,8]. In the present study,18.7% of all OSCC occurred in young patients. There is considerable geographic variation in the incidence of OSCC in the young population, with comparatively lower incidence in western countries than India where estimated incidence is 16-28% [2,3]. This is probably because, with cigarette/beedi smoking and tobacco/pan/betel quid chewing being a common habit in India, the proportion of young population getting early and habitual exposure to these carcinogenic products is higher [2,9]. Factors responsible for risk factor abuse by young population include adverse habits by parents and siblings, peer group pressure, easy access and low cost of tobacco products and aggressive advertising [9].
Tobacco usage (smoke and smokeless forms) and alcohol consumption are regarded as the major risk factors for OSCC [2,9,10]. Further, alcohol being a cancer promoter has a synergistic effect with smoking [10]. People practicing all the latter habits together had 123 fold greater incidence of OSCC compared to abstainers [10]. Up to 85.7% of young and 90.1% of older patients in the current study were habituated to tobacco usage and/or alcohol consumption, confirming the essential carcinogenic role of these traditional risk factors.
Considering that the pathogenesis of OSCC is complex and multifactorial, many studies have tried to investigate the putative risk factors for OSCC in young patients. In the present study, both the groups were almost equally exposed to adverse habits (85.7% in young and 90.1% in older patient) and there was no statistically significant difference in the occurrence of OSCC in young and older subjects with risky habits (p=0.984). The latter findings are in concordance with studies conducted by Acharya S and Tayaar AS, Beena VT et al., Abdulla R et al., and Iamaroon A et al., [2,7,9,10]. Even though, the latter studies have demonstrated that the same risk factors are present in both the age groups, other factors including genetic susceptibility, socioeconomic status, occupational carcinogen exposure, immune status, use of immuno-suppressive medications and previous human papilloma viral infections should be analysed in the young patients [2,3].
Similar to study by Beena VT et al., where family history of OSCC was more frequent in young subjects (8.2%), as compared to older subjects (0.1%), 5.7% of our young patients had family history in contrast to 0.7% in the older patients [7]. Thus family history of OSCC could be a risk factor in the young subjects (p=0.031). Male predominance was found in both the age groups, which corroborated with most of the earlier studies [2,7-10].
With regard to the tumour site, majority of the studies have found predilection for tongue in the young patients [3]. Abdulla R et al., found that, tongue (29%) followed by buccal mucosa (27.9%) and alveolus (13.9%) were the common sites in the young in contrast to older patients where buccal mucosa (32.1%) followed by alveolus (16.4%) and tongue (16.1%) were the common sites [9]. Acharya S et al., reported buccal mucosa (47%), alveolar process (24%) and tongue and floor of mouth (23%) as the common sites in young and alveolar process (42%), buccal mucosa (37%) and tongue and floor of mouth (14%) as major sites in older patients [2]. In this study, buccal mucosa was the major site in both age groups followed by tongue (20%) and alveolar ridge (17.1%) in the young and alveolar ridge (17.1%) and tongue (13.8%) in the older. This discrepancy could be due to varying sample size among studies and the most frequent risky habit in the studied subjects [3]. There was no statistically significant difference in the anatomic site distribution pattern between the two groups in the present study (p=0.58).
In both the age groups, OSCC had predominant exophytic morphology, which is in concordance with another south Indian study where 62% and 72% of OSCC presented as exophytic lesions in the young and older subjects respectively [2].
Majority of the OSCC, in both age groups, were moderately differentiated [Table/Fig-4] which is in concordance with other Indian studies [7-9]. There was no significant difference with respect to tumour differentiation between the age groups, in this study as well as the latter studies [Table/Fig-4,6].
(a) Well differentiated squamous cell carcinoma with numerous keratin pearls; (b) Moderately differentiated squamous cell carcinoma with few areas of keratinisation; (c) Poorly differentiated squamous cell carcinoma with barely identifiable squamous differentiation (H&E, 200x).
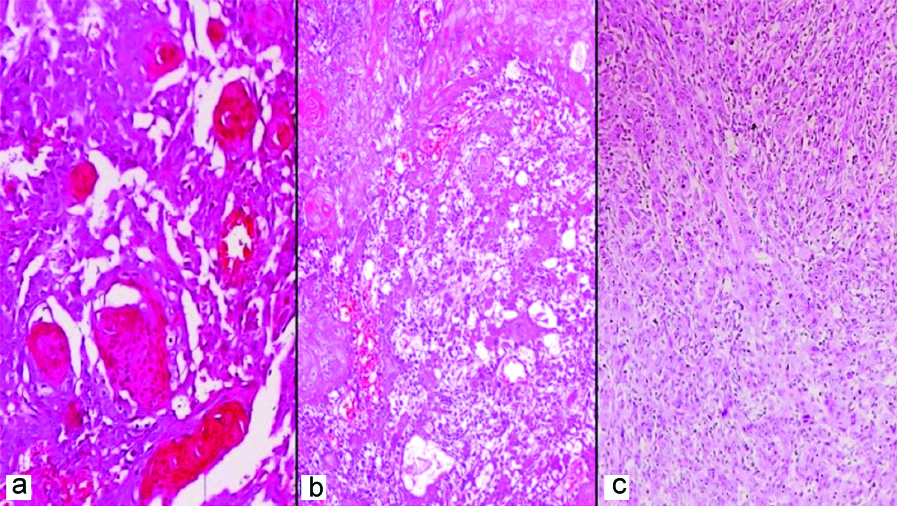
In the present study, the frequency of perineural invasion was relatively similar in the two age groups (8.6% in young and 9.2% in older subjects). Other adverse prognostic indicators like lympho-vascular invasion, extranodal extension and WOPI were relatively more frequent in the young compared to older group, suggesting a possibility of OSCC being more aggressive and infiltrative in the young subjects. However, a statistically significant correlation between these prognostic indicators and the age groups was not observed [Table/Fig-4]. According to a systematic review, that evaluated 340 studies on OSCC in young patients, most of the authors have concluded that OSCC is more aggressive in young adults [3].
WOPI-5 is assessed at advancing tumour edge and is defined as “tumour dispersion of ≥1 mm between tumour satellites” [5]. In order to simplify prognostication AJCC recommends only one cut-point for evaluation of pattern of invasion i.e., presence/absence of WOPI-5 [5]. Multivariate analyses have validated WOPI-5 as an outcome predictor for OSCC. It predicts loco-regional recurrence and disease specific survival [5]. There is paucity of studies evaluating WOPI in OSCC. In the current study, WOPI-5 was present in 8.6% of OSCC in young and 6.5% of OSCC in older subjects. However, this difference was not statistically significant between the age groups (p=0. 676).
The frequency of lymph node metastasis was relatively higher in young (40%) compared to older patients (27.6%), however this difference between the age groups was not statistically significant. [Table/Fig-7]. Studies have shown that incidence of regional nodal metastasis is higher in the younger subjects with a tendency for early lymphatic spread [8,11].
(a) Lymph node with metastatic tumour (arrow indicates tumour) (H&E, 40x); (b) Perineural invasion with nerve twig (long arrow indicates nerve) and tumour cells (short arrow indicates tumour cells) infiltrating the perineural space. (H&E, 200x); (c) WOPI with dispersed tumour satellites (arrows indicate tumour satellites) at advancing tumour edge (H&E, 100x).
WOPI: Worst pattern of invasion
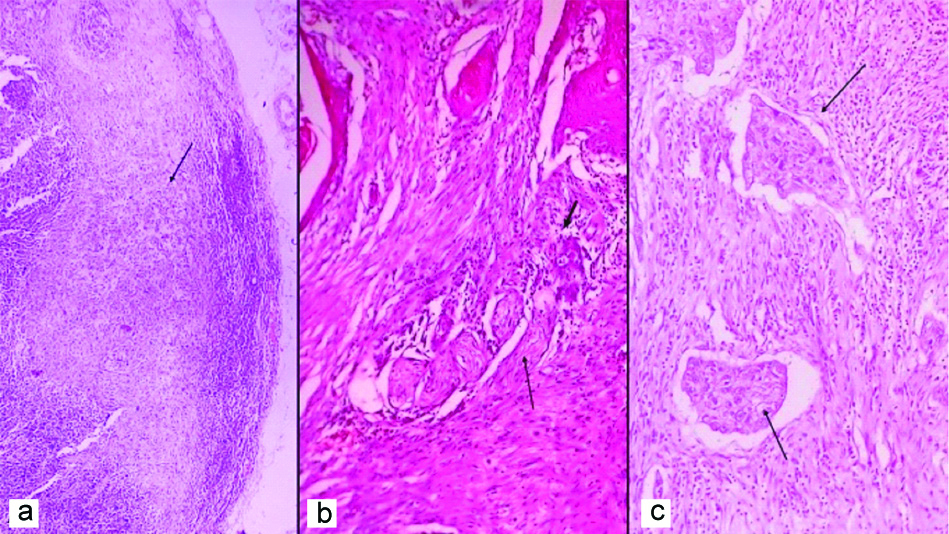
Regardless of age, most patients were diagnosed in advanced stage (stages III and IV), which corroborate with findings of previous Indian studies and studies conducted abroad [6,9,10,12,13]. Such presentation is probably due to delayed diagnosis or delay in doctor consultation and lack of dedicated screening programs for pre-malignant and early lesions of OSCC [9]. In many studies, which analysed OSCC in young, greater prevalence of OSCC was found at advanced stages suggesting a more aggressive biological behaviour in young patients [3]. Regarding TNM stage, Benevenuto TG et al., reported a statistically significant correlation between young and old patients (67% of OSCC patients, in stages III and IV, were young) [3, 14]. In this study, even though we found a relatively higher frequency of advanced stage presentation in young compared to older subjects (77.2% vs. 68. 4%), this association was not of statistical significance (p=0.31).
Limitation(s)
The limitations of the study are the relatively smaller number of cases in the younger age group and lack of follow-up.
Conclusion(s)
Our frequency of OSCC among young subjects largely corresponds to the frequencies described in other Indian studies. Young patients, have higher family antecedent of OSCC. Clinical parameters like risk factor exposure, sex distribution and some tumour characteristics like commonest anatomic site, gross appearance, tumour differentiation and perineural invasion are relatively similar between the two age groups. Indicators of aggressive biological behaviour like lymphovascular invasion and lymph node metastasis, extranodal extension, WOPI and presentation at advanced TNM stage are relatively commoner, though not statistically significant, in the young compared to older group, suggesting a possibility of OSCC exhibiting a more aggressive behaviour in the young patients. Advanced stages with increased disease burden and adverse prognosis denotes the need for early detection of the disease. Intensive awareness and screening programmes should be instituted especially in developing countries. Further in-depth studies with larger sample size and disease-free survival and follow-up data, are required to determine the specific tumour characteristics, biological behaviour and prognostic indicators of OSCC in young patients.
OSCC: Oral squamous cell carcinoma; p-value <0.05 to be considered significant
p-value as determined by chi-square test; TNM: Tumor node metastasis; p-value <0.05 to be considered significant