Gastric cancer is a malignant tumour with a fifth position amid all cancers and its mortality rate is third, globally [1]. But the frequency varies in several parts of the world. In India, amongst men it is the fifth most common cancer and in women it is in seventh position [2]. Multifactorial causation has an important role in gastric cancer which includes various dietary and environmental factors.
The most common distant mode of metastasis is PD. In such cases, a gastrectomy does not improve the prognosis but can rather hamper the patient’s quality of life [3]. Also, PD is the most common recurrence pattern after curative gastrectomy with extended lymphadectomy [4]. Computed Tomography (CT) scan, Positron Emission Tomography (PET) and laparoscopic evaluation are commonly used to detect PD but they are either inconvenient or overly complex, so surgeons face to unforeseen non-curative operation [5]. Serum tumour markers are usually used for prompt diagnosis of tumour burden and occult metastases in variety of cancer patients. CA125 is an antigenic determinant detected by a murine monoclonal antibody OC125 [6]. Initially, it has been used primarily as a tumour maker for ovarian cancer. However, many new studies suggest that the peritoneum is an important source of CA125 and that there is a positive correlation between serum CA125 level and PD in cases of malignant gastric neoplasm [7-9]. Advanced gastric cancer with PD has poor prognosis and so early detection is necessary. The objective was to study the clinical significance of serum CA125 and its relation to PD in cases of gastric cancer.
Materials and Methods
This cross-sectional observational study was conducted for two years from January, 2018 to January, 2020 on 120 cases at Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal (India). Approval from the Institutional Ethics Committee (Registration No. ECR/35/Inst/WB/2013) and written informed consent from the patients was taken.
Inclusion criteria: All confirmed cases of malignant gastric neoplasm by endoscopic biopsy planned for gastrectomy and preoperative serum CA125 level done in confirmed malignant gastric neoplasm cases were included in the present study after taking proper informed consent.
Exclusion criteria: Cases with no preoperative serum CA125, received prior chemo-irradiation, critically ill patients unfit for surgery and those refused to give consent for the study were excluded.
Proper history, clinical examination, routine investigation was done according to the proforma. Rapid Urease Test (RUT) was also performed. Peripheral blood sample for CA125 assay was collected one week prior to surgery. The sera were analysed using an immunoradiometric assay (ovarian cancer antigen (OD289), Omega Diagnostics Ltd., UK). The cut-off value of CA125 was 35U/mL according to the manufacturer’s manual. This data was to be compared among the same patients diagnosed with PD. PD was identified through USG/CT or intraoperatively during open surgery. All the gastrectomy specimens were sent for gross examination, staging and histopathology. The paraffin embedded tissue blocks were subsequently stained for H&E.
Statistical Analysis
Microsoft excel 2016 and Statistical Package for the Social Sciences (SPSS) version 18 (SPSS, Inc. Chicago, IL, USA) was used for data analysis. Statistical forms were used to record the demographic, clinical, laboratory data of each patient. Chi-square test was used for the comparison between the groups. Categorical values were expressed as rate with 95% Confidence Intervals (CI). A p-value of <0.05 was considered significant. The sensitivity, specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) were used for elective assessment.
Results
This study comprised 120 cases. Patients in the age group of 51-60 years were most commonly affected [Table/Fig-1]. Mean age of presentation was 54.3 years. Men (84 cases, 70%) were affected mostly then women (36 cases, 30%). The male to female ratio was 2.3:1.
Age and sex-wise distribution of cases (N=120).
| Age (in years) | Male | Female | Total | Percentage (%) |
|---|
| 31-40 | 6 | 5 | 11 | 9.2 |
| 41-50 | 17 | 11 | 28 | 23.3 |
| 51-60 | 39 | 10 | 49 | 40.8 |
| 61-70 | 19 | 9 | 28 | 23.3 |
| 71-80 | 1 | 1 | 2 | 1.7 |
| 81-90 | 2 | 0 | 2 | 1.7 |
| Total | 84 | 36 | 120 | 100 |
| Percentage (%) | 70 | 30 | | |
Majority of the patients complained of anorexia (82 cases, 68%) followed by dyspepsia (60 cases, 50%), pallor (60 cases, 50%) and hematemesis (52 cases, 43%). Consumption of fermented fish/red meat was seen in 75 cases (62.5%) followed by smoking seen in 68 cases (56.7%) [Table/Fig-2]. Family history was present only in 2 cases. Of the total cases, RUT results showed positivity in 14 cases (11.6%). Partial gastrectomy (77 cases, 64.2%) was the most common specimen received [Table/Fig-2]. Antrum (56 cases, 46.6%) was the most common site involved followed by the body (24 cases, 20%) [Table/Fig-2].
Clinicopathological characteristics of the cases.
| Category | Subcategory | Number of cases (%) |
|---|
| Clinical complaints | Anorexia | 82 (68) |
| Dyspepsia | 60 (50) |
| Pallor | 60 (50) |
| Hematemesis | 52 (43) |
| Dietary history | Smoking | 68 (56.7) |
| Alcohol | 43 (35.8) |
| Fresh fruits/vegetables | 20 (16.7) |
| Fermented fish/meat | 75 (62.5) |
| Types of gastrectomy specimens | Partial | 77 (64.2) |
| Total | 35 (29.2) |
| Subtotal | 6 (5.0) |
| Total gastrectomy with splenectomy | 2 (1.6) |
| Sites of stomach involved | Antral-pyloric | 17 (14.2) |
| Antrum | 56 (46.6) |
| Pylorus | 17 (14.2) |
| Body | 24 (20.0) |
| Cardia | 3 (2.5) |
| Fundus | 3 (2.5) |
| Gross features (Borrmann classification) [10] | Polypoid (Type 1) | 57 (47.4) |
| Ulcerative (Type 2) | 41 (34.2) |
| Infiltrative ulcerative (Type 3) | 11 (9.2) |
| Diffuse infiltrative (Type 4) | 11 (9.2) |
| WHO classification of Adenocarcinoma (2019) [10] | Tubular (solid)-poorly differentiated | 50 (41.6) |
| Tubular-moderately differentiated | 34 (28.3) |
| Tubular-well differentiated | 12 (10.0) |
| Mucinous | 12 (10.0) |
| Papillary | 8 (6.7) |
| Signet ring cell type | 4 (3.4) |
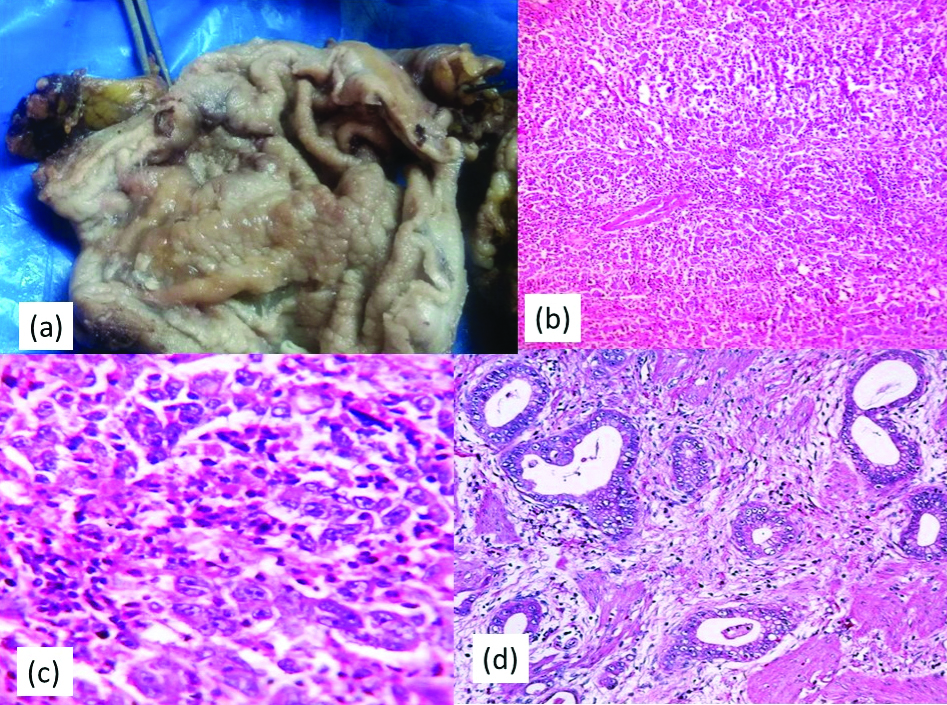
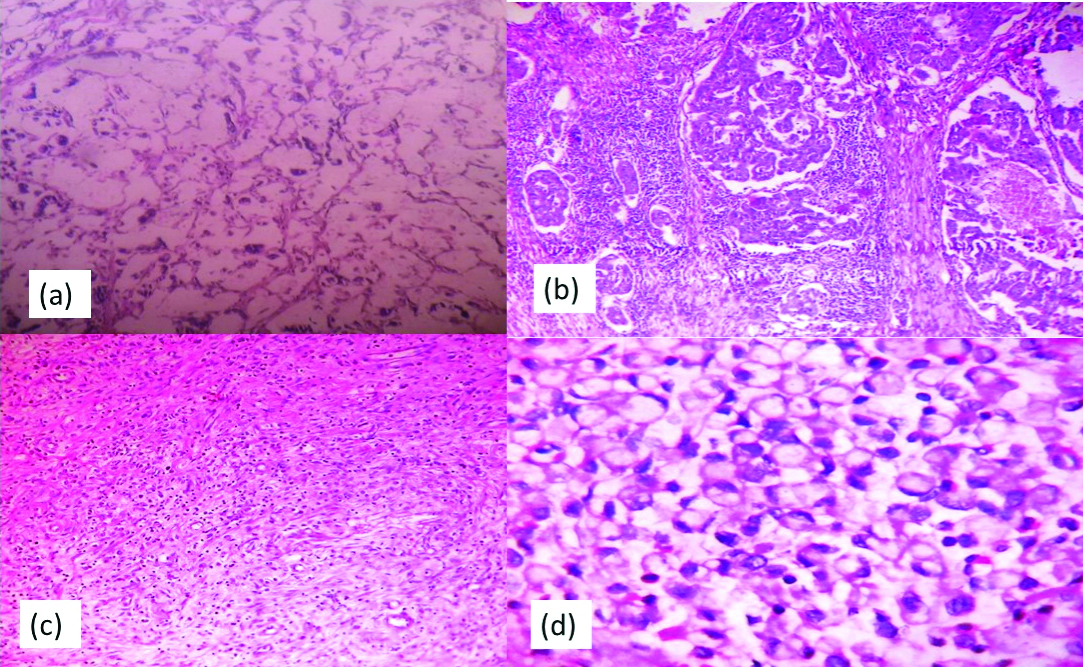
Gross features or the macroscopic appearances of the gastric cancer were subclassified using Borrmann classification [10] which includes four types. Polypoid (57 cases, 47.4%) was the most common gross feature followed by ulcerative (41 cases, 34.2%) [Table/Fig-2,3a]. Rest of them were infiltrative ulcerative and diffuse infiltrative. According to the WHO classification (2019) of gastric tumour [10], we have categorised adenocarcinoma [Table/Fig-2]. Tubular-poorly differentiated (50 cases, 41.6%) [Table/Fig-3b,c] comprises the majority of the cases followed by tubular-moderately differentiated (34 cases, 28.3%) [Table/Fig-3d]. Tubular-well differentiated, mucinous adenocarcinoma [Table/Fig-4a], papillary adenocarcinoma [Table/Fig-4b] were also diagnosed. Signet ring cell type [Table/Fig-4c,d] was the least common type.
Gross specimen of stomach: (a) Showing ulcerative type of growth. Case of tubular-poorly differentiated adenocarcinoma; b) Section shows solid pattern of growth and poorly formed glands (x100, H&E); c) Section shows pleomorphic hyperchromatic cells with vesicular chromatin and prominent nucleoli (x400, H&E). Case of tubular-moderately differentiated adenocarcinoma; d) Section shows gland formation lined by pleomorphic hyperchromatic cells (x100, H&E).
Case of mucinous adenocarcinoma: a) Section shows malignant cells floating in pools of mucin (x100, H&E). Case of papillary adenocarcinoma; b) Section shows papillae formation with fibrovascular connective tissue core (x100, H&E). Case of signet ring cell type adenocarcinoma; c) Section shows poorly cohesive signet ring type of cells (x100, H&E); d) Section shows signet ring cells with eccentric nucleus and pools of mucin (x400, H&E).
TNM staging was done. T3 stage (70 cases, 58.3%) comprised the major bulk of the cases followed by T2 (32 cases, 26.7%), T4 (10 cases, 8.3%) and T1 (8 cases, 6.7%). Likewise, N2 nodal staging (72 cases, 60%) was leading followed by N0 (34 cases, 28.4%), N1 (10 cases, 8.3%) and N3 (4 cases, 3.3%). Distal metastasis consisting 43.3% (52 cases) of the cases with liver (25 cases, 48.1%) as the most common site of metastasis followed by PD (22 cases, 42.3%), left supraclavicular lymph node (3 cases, 5.8%), and multiple metastasis (2 cases, 3.8%). Majority of the cases were stage IIIA (59 cases, 49.2%) and stage IV (52 cases, 43.3%) [Table/Fig-5].
Frequency of overall staging.
| Stage | Number of cases (%) |
|---|
| IA | 1 (0.8) |
| IB | 1 (0.8) |
| IIA | 2 (1.6) |
| IIB | 1 (0.8) |
| IIIA | 59 (49.2) |
| IIIB | 3 (2.7) |
| IIIC | 1 (0.8) |
| IV | 52 (43.3) |
For PD, CA125 cut-off value ≥35 U/mL was used as a reference value [7]. CA125 had a very high odds ratio of 27 to assess peritoneal involvement. There was a significant correlation (p-value <0.0001) with PD and CA125 serum assay [Table/Fig-6]. The Chi-square test was 41.9 and degree of freedom as 1. The statistical values are shown in [Table/Fig-7].
Diagnostic ability of CA 125 serum marker.
| CA 125 (U/mL) Serum assay | Peritoneal Dissemination (PD) | Odds ratio | 95% CI* | p-value |
|---|
| Present | Absent |
|---|
| ≥35 U/mL | 18 | 14 | 27 | 7.95-91.66 | <0.0001 |
| <35 U/mL | 4 | 84 |
*95% CI: 95% Confidence interval, Chi-square value was 41.9; CA 125: Cancer antigen 125 p-value <0.05 to be considered significant
| Statistic | Value | 95% CI* |
|---|
| Sensitivity | 81.8% | 59.72-94.81 |
| Specificity | 85.7% | 77.19-91.96 |
| PPV | 56.2% | 43.24-68.46 |
| NPV | 95.4% | 89.61-98.08 |
| Accuracy | 85.0% | 77.33-90.86 |
*95% CI: 95% confidence interval; PPV: Positive predictive value; NPV: Negative predictive value
Discussion
Gastric cancer is one of the most commonly observed malignant tumors. Incidence and the patterns of gastric cancer show a worldwide variation. High incidence is seen in countries of Southeast Asia, Japan and China as compared to India [11]. Among 120 cases, the mean age of presentation was 54.3 years with male to female ratio of 2.3:1. This was quite similar to the other studies [Table/Fig-8]. Majority of the patients complained of anorexia (68%) followed by dyspepsia (50%), pallor (50%) and hematemesis (43%). These clinical findings were comparable to the other studies [2,12-14]. Family history was present only in 2 cases. This estimate was low due to inadequate history given by the patients. Consumption of fermented fish/red meat was 62.5% followed by smoking (56.7%). This was coherent with other studies [2,14]. As per RUT results, history of preceding H. pylori infection was seen in 11.6% of cases. Nandi A et al., RUT results showed 24.1% positivity [12].
Age and sex incidences, most common site affected and TNM stage compared to other studies [2,12-14].
| Name of the study | Mean age (years) | Male:Female ratio | Most common site affected | TNM staging |
|---|
| Barad AK et al., [2] | 58.2 | 2.2:1 | Antrum | IV |
| Nandi A et al., [12] | 53.02 | 2.4:1 | Antrum | III |
| Shirin L and Rahman MM [13] | 52.89 | 3:1 | Pyloric | IV |
| Safaee A et al., [14] | 59.7 | 2.5: 1 | Pyloric | IV |
| Present study | 54.3 | 2.3:1 | Antrum | III |
The most common site affected was antrum (46.6%). This was compared to other studies [Table/Fig-8]. Polypoid (Borrmann type 1) (47.4%) was the most common gross feature so as in the study done by Nandi A et al., [12]. The maximum palliative surgery was partial gastrectomy. Similar findings were seen with other studies [12,14,15]. Among the adenocarcinoma, the most common type was tubular (solid)-poorly differentiated. This was consistent with other studies [2,12-14]. According to the TNM staging, stage III (49.2%) was among the majority followed by stage IV (43.3%) as compared to the other studies [Table/Fig-8].
Gastric carcinomas are a biologically and genetically heterogenous group of tumours with multifactorial aetiologies. It occurs mostly in persons aged 30 years or more and incidence increases progressively with age in both men and women [16]. The common aetiology is smoking, dietary factors such as high intake of smoked meat, salt preserved food and low intake of fresh fruit and vegetables, H. pylori infection [17]. Histological types of adenocarcinoma are subclassified into different categories as tubular composed of slit-like and branching tubules, papillary has elongated finger-like supported by fibrovascular connective tissue cores, mucinous composed of extracellular mucin pools (>50%), signet ring cell type characterised by cytoplasmic mucin with an eccentrically placed nucleus and mixed is composed of glandular and signet ring/poorly cohesive cellular components. CK7, CK20, MUC6, CDX2 are specific for primary gastric adenocarcinomas [18].
The prognosis for advanced gastric cancer especially with PD is poor and the quality of life is also compromised. So, early diagnosis of the degree and extent of PD is essential. Nowadays, USG/CT is used to assess the PD. The specificity is high but the sensitivity is low and with a drawback in finding the nodules less than 5 mm [19]. Laparoscopic examination is well-precise but it is an invasive procedure. Nonetheless, current serum tumour markers can be easily and cost-effectively identified and can be useful for the preoperative staging of neoplasms, postoperative monitoring of treatment and early diagnosis of recurrence.
Tumour marker is selectively secreted by cancer cells alone, in the blood or in other body fluids. CA125 is a repeating peptide epitope of the mucin MUC16 and is identified as a 5797-base pair cDNA isolated from the OVCAR-3 cDNA library [20]. Promoting cancer cell proliferation and inhibiting anticancer immune responses are its biological function. CA125 is formerly found as a distinctive biological marker for ovarian cancer and is considered to be a method for detection of gastric cancer [21]. CA125 is also related to ovarian, uterine, lung and pancreatic cancers [22]. Some studies have shown that Systemic Inflammatory Response (SIR) and fibrinogen also plays an important role in tumour progression and metastasis in advanced gastric cancer [23-25].
For PD, CA125 cut-off value ≥35 U/mL was used as a reference value. The sensitivity and specificity of CA125 was compared with other studies [Table/Fig-9] [13,26-29]. Emoto S et al., showed sensitivity of 46% [30]. CA 125 had the highest odds ratio 27(95% CI: 7.95-91.66) for predicting PD. Similarly, to Shirin L and Rahman MM (18.3, 95% CI: 4.39-76.64) [13], Hwang GI et al., (24.46, 95% CI: 11.17-53.37) [28] and Nakata B et al., (14.56, CI: 3.23-65.5) [7]. The PPV and NPV were 56.2% and 95.4%. This was comparable with the studies done by Shirin L and Rahman MM (PPV: 65%, NPV: 91%) [13] and Chen C et al., (PPV: 83.3%, NPV: 62.6%) [26]. Most gastric cancer are asymptomatic and so most cases present at late stage. Peritoneal metastasis is one such event and in such cases, a gastrectomy does not improve the prognosis but can rather hamper the patient’s quality of life. Hence, the need of a diagnostic tool which could correctly predict peritoneal metastasis in gastric cancer is important.
Comparison of the sensitivity and specificity of CA125 with other studies [13,26-29].
| Name of the study (year of study) | Sensitivity (%) | Specificity (%) |
|---|
| Shirin L and Rahman MM (2016) [13] | 73 | 86 |
| Chen C et al., (2017) [26] | 28.7 | 99 |
| Haung C et al., (2019) [27] | 79.1 | 84.9 |
| Hwang GI et al., (2004) [28] | 38.6 | 98.4 |
| Fujimura T et al., (2002) [29] | 55 | 100 |
| Present study | 81.8 | 85.7 |
CA125: Cancer antigen 125
Limitation(s)
Firstly, the assessment of postoperative level of serum CA125 for recurrence patterns of gastric cancer was not done. Secondly, small sample size for shorter period of time and thirdly, only one serum marker CA125 was evaluated as the other serum markers like CA 72-4, CA 19-9, CA 15-3 were not routinely tested for gastric carcinoma patients in our hospital.
Conclusion(s)
Gastric cancer is one of the most common cancers worldwide and remains to be an important malignant disease with significant geographical, ethnic and socio-economic difference in distribution. Majority of malignant gastric neoplasms are asymptomatic and hence, presents in late stage. Early detection of peritoneal disease will help the clinicians to stratify the treatment of gastric cancers and to decide whether to go for surgical or palliative intervention. Higher sensitivity suggests that preoperative serum CA125 is a promising tool to predict PD which aids in subsequent alternative mode of treatment, lesser morbidity and better survival of patients.
*95% CI: 95% Confidence interval, Chi-square value was 41.9; CA 125: Cancer antigen 125 p-value <0.05 to be considered significant
*95% CI: 95% confidence interval; PPV: Positive predictive value; NPV: Negative predictive value
CA125: Cancer antigen 125