Introduction
COVID-19 is caused by coronaviruses, that are enveloped, non-segmented, positive-sense RNA viruses. These belong to the subgenus sarbecovirus and orthocoronovirinae subfamily [1]. SARS-CoV-2 is the seventh addition to the family of viruses that can infect humans. MERS-CoV (Middle Eastern Respiratory Syndrome Corona Virus) and SARS-CoV belong to the same family and caused the Middle East Respiratory Syndrome in 2012 and severe acute respiratory syndrome in 2002/2003, respectively [2,3]. SARS-CoV-2 was first detected as an epidemic in Wuhan, China and has since then spread across the globe [4]. It has been found that human-to-human transmission occurs via respiratory droplets, respiratory secretions and direct contact of an infected individual [5,6]. It may lead to a variety of clinical features, ranging from an asymptomatic carrier to a severe pneumonia leading to acute respiratory distress and multi-organ dysfunction [7]. The virus has been so rapid in its spread that it was characterised as a pandemic by the World Health Organisation (WHO). As of 4th September, 2020, the global count of confirmed cases and deaths stand at 26,121,999 and 8,64,618, respectively [8]. This article aimed to perform a review of current management and therapeutic options for COVID-19 that have been tried in various parts of the world.
Management
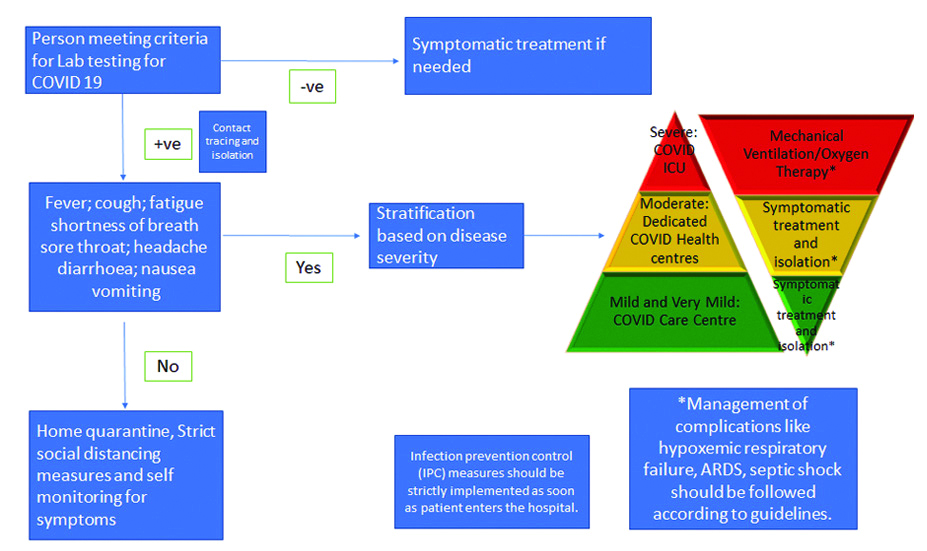
Once diagnosed, most patients do not require any treatment due to their mild symptoms. Wu Z and McGoogan JM conducted the largest cohort study having 44415 patients in China and found that 81% people had mild disease (mild symptoms up to mild pneumonia), 14% had severe features (dyspnoea, hypoxia, or >50% lung involvement on imaging) and only 5% had critical symptoms (respiratory failure, shock, or multi-organ system dysfunction) [9]. Currently, during the ongoing pandemic, treatments around the world focus on managing the specific symptoms associated. A summary of management has been provided in [Table/Fig-1] [10-12].
Summary of COVID-19 management [10-12].
ARDS: Acute respiratory distress syndrome
Asymptomatic Patients
Asymptomatic patients/contacts who have been tested positive are advised for home isolation. The main goal while isolating the patient at home is to watch for development of any symptoms like difficulty in breathing, dip in O2 saturation etc. The other important thing to take care of is Infection Prevention and Control (IPC). For home settings, this would include limiting the patient’s movement inside the house, minimising any shared spaces, no entry into the room of patients and maintaining safe distance. Also, other measures like limiting visitors, proper hand hygiene, wearing a medical mask and avoiding any contact with patients’ fluids should be taken care of. Finally gloves should be used, patient’s laundry should be in a different bag, dedicated linen should be used and frequently used surfaces should be disinfected regularly. The release of asymptomatic patients from home isolation has been advised to be 10 days after testing positive, as per the WHO [13]. Similarly, Indian guidelines advise for discharge after 10 days of symptom onset and if there is no fever for three days. Further monitoring of health should continue for seven days. Specific guidelines for home isolation have been provided by the Indian government [14].
Management of Mild COVID-19
Most patients with COVID-19 present with mild symptoms and their management can be easily handled based on certain other factors. These are majorly the clinical presentation, risk factors, requirement for supportive care and the possibility of isolating at home [15]. The primary method in such cases would be isolation to prevent further spread of disease. This may be done at home, a community health facility or a dedicated isolation facility. The possibility of co-infection and consideration about prevalent febrile illnesses in the area should be regarded. Along with this, supportive care should be given to the patient which may include antipyretics for fever and pain, adequate nutrition and hydration [12]. Despite the earlier inconclusive reports about ibuprofen related to COVID-19, currently there is no recommendation against use of Non Steroidal Anti-Inflammatory Drugs (NSAIDs) [16]. Along with this, the patients must be well-informed of the complications and worsening symptoms (breathlessness, light headedness etc.,) and as to when they must seek medical care. Also, Hydroxychloroquine (HCQ) can be considered in high-risk patients under medical supervision (>60 years age or having co-morbidities) [12]. Currently, there is no recommendation for use of antibiotics for treatment or prophylaxis [17]. Finally, the National Institute of Health (NIH) has mentioned that there is insufficient data to advise for or against the use of antivirals or immunomodulatory therapy in mild cases [18].
Management of Moderate COVID-19
The management of moderate COVID-19 remains similar to the management of mild cases except for the higher requirement of close monitoring of these patients due to greater chances of worsening. As per WHO, moderate cases are classified on the basis of development of pneumonia (fever, cough, dyspnoea or fast breathing), but not severe pneumonia with SpO2 >90% [17]. The NIH, on the other hand, defines moderate illness as evidence of lower respiratory tract illness by clinical evaluation or imaging, with SpO2 >94% at room air [18]. They even recommend the importance of identifying bacterial pneumonia in these cases and subsequent treatment for the same. On a case-to-case basis, it might be important to admit moderate cases considering their risk of worsening, clinical status and risk factors. Also, like in mild cases, there is insufficient data to recommend the usage of antivirals or immunomodulatory therapeutics [18]. According to latest Indian guidelines, apart from following same protocol as in mild cases, prophylactic dose of unfractionated heparin or Low Molecular Weight Heparin (LMWH) may be given and systemic corticosteroids may be considered if condition is worsening in terms of oxygen need or inflammatory markers. Also, hydroxychloroquine, remdesivir and convalescent plasma may be administered [12].
Management of Severe and Critical COVID-19
Severe cases of COVID-19 as per WHO, have severe pneumonia (clinical signs of pneumonia with one of the following: Respiratory rate >30/min; severe respiratory distress; or SpO2 <90%) and need urgent management [17]. The NIH has defined severe illness as SpO2 ≤94% on room air at sea level, respiratory rate >30/min, PaO2/FiO2 <300, or lung infiltrates >50% [18]. The main deciding factor for management is worsening respiratory function. It is expected that institutes treating these patients should have readily available equipment for measurement of oxygen saturation and maintain regular flow of oxygen like venturi mask, pulse oximeters etc., [18]. The management of severe disease mainly focuses on the management of complications like shock, respiratory failure, thromboembolism, cardiovascular complications and involvement of other systems (renal, vascular, cardiac, gastrointestinal) [15]. The main measure of management of severe disease is providing rapid airway management and oxygen therapy. After stabilising the patient, oxygen is delivered to maintain SpO2 >90% in non-pregnant women and >92-95% in pregnant women. Other helpful techniques may include positioning techniques like high supported sitting and prone positioning which may help to optimise oxygenation and ease breathlessness [19]. These patients should also be constantly monitored for airway clearance. A special consideration should be on the amount of fluids as excessive fluids may worsen oxygenation [20].
Finally, a constant evaluation should be made for these patients using regular blood tests (including markers like C-Reactive Protein (CRP), D dimer and ferritin), pulmonary function tests, Electrocardiography (ECG) and imaging techniques. These help to detect clinical deterioration for further management [11,17,18]. According to the latest Indian guidelines, anticoagulation should be provided if patient has no high-risk of bleeding, corticosteroids should be used (methylprednisolone 1-2 mg/kg or dexamethasone 0.2-0.4 mg/kg) for 5-7 days and tocilizumab can be considered [12]. Critical COVID primarily includes the development of Acute Respiratory Distress Syndrome (ARDS), shock and septic shock [17] and the management of these should follow already established international standards [11,12,17,21,22]. A brief summary of management of complications has been given in [Table/Fig-2] [12,22-29].
Management of complications [12,22-29].
| Complications | Management |
|---|
| Respiratory: Pneumonia, ARDS, Respiratory failure | Use of supplemental oxygen when fall in saturation is seen with a target SpO2 of >94%, using empirical antibiotics, early recognition of ARDS, use of mechanical ventilation at lower tidal volume and lower inspiratory pressures, use of prone ventilation, conservative usage of fluids and following established protocol [12,22]. |
| Cardiac: Cardiomyopathy, Arrythmia, Myocardial injury | Should focus on precipitating reversible causes of damage like electrolyte imbalance, a separate heart-lung team should be called early to prevent damage [23]. |
| Renal: Acute Kidney Injury (AKI) | Using KDIGO [24] guidelines, managing volume overload and barotrauma to reduce risk of new AKI due to haemodynamic changes and cytokine storm and considering renal replacement therapy and extracorporeal options when needed as per assessment [25]. |
| Nervous: Polyneuropathy, myopathy, Encephalopathy, Cerebrovascular disease, GBS | Management should follow established protocols for specific complication but with full precautions due to infection risk during invasive procedures and giving thrombolytic therapy etc., [26] |
| Gastrointestinal: Liver injury [27] | Mostly self-limited in mild cases. Resolve with resolution of COVID. In severe cases, specific treatment may be needed [28] |
| Vascular: Hypercoagulability, DIC, Multisystem inflammatory syndrome in children, Septic shock | Early prophylaxis for venous thromboembolism may be warranted and therapy may need to be continued post-discharge. If patients develop thromboembolism, anticoagulant therapy may be used as per protocols. Management of other complications as per standard protocols [12,29] |
ARDS: Acute respiratory distress syndrome; GBS: Guillain-Barré syndrome; DIC: Disseminated intravascular coagulation; AKI: Acute kidney injuries; KDIGO: Kidney disease improving global outcomes
The major goal should be to prevent complications by using prophylactic LMWH or heparin for thromboembolism, turning patient every two hours to prevent pressure ulcers, giving early enteral nutrition and use of Proton Pump Inhibitors (PPIs) to reduce risk of stress, ulcers or Gastrointestinal (GI) bleed and early mobilisation of patient to prevent weakness.
Therapeutics and Vaccines
As of 3rd September,2020,, no drug or vaccine has been approved by the Food and Drug Administration (FDA) for the treatment or prevention of COVID-19 except for emergency use of remdesivir in severe cases [15,30-32]. Here, a summary of some of the available therapeutic options have been presented which have shown or may show promising results [Table/Fig-3] [12,33-39].
Summary of therapeutics [12,33-39].
| Mechanism of action | Major side effect(s) | Dose |
|---|
| Hydroxychloroquine | Inhibits viral entry and endocytosis by multiple mechanisms and has host immunomodulatory effects | QT prolongationRetinopathy | 400 mg BD on day 1 and then 200 mg for 4 days in moderate cases [12]For prophylaxis of health care workers: 400 mg twice a day on day 1, followed by 400 mg once weekly for next 7 weeksFor prophylaxis for asymptomatic contacts: 400 mg twice a day on Day 1, followed by 400 mg once weekly for next three weeks [34] |
| Convalescent plasma/serum | Antibodies may cause viral neutralisation, other mechanisms like Antibody dependant cellular cytotoxicity (ADCC) may be possible [35] | FeverAllergic reactionOther transfusion related side effects [36] | Ranging from 4 to 13 mL/kg [12] |
| Remdesivir | Inhibits viral RNA dependant RNA polymerase | GI intoleranceHepatotoxicity | 200 mg IV on day 1 and then 100 mg IV daily for 4 days [12] |
| Lopinavir/Ritonavir | Inhibits 3-chymotrypsin like protease preventing viral protein generation | QT prolongationHepatotoxicity | Most commonly used and studied dose is 400 mg/100 mg twice daily for up to 14 days [33,37] |
| Oseltamivir | Neuraminidase inhibitor for influenza | GI IntoleranceHeadacheInsomnia | 150 mg BD for 5 days |
| IVIg | Antibodies may cause viral neutralisation, other mechanisms like ADCC maybe possible | Can interact with other anti-virals | 0.3 to 0.5 g/kg/d for 5 days in a trial [38] |
| Ivermectin | May be nuclear import of viral proteins [39] | PruritisLymphadenitisArthralgia | Planned 12 mg/week for a trial |
| Tocilizumab | IL-6 blocker | Hepatotoxic, Infusion-related reaction, Increased cholesterol | 8 mg/kg given slowly in 100 mL NS over 1 hour; can be repeated once after 12-24 hours [12] |
| Corticosteroids | Methylprednisolone (0.5 mg to 1 mg/kg) for 3 days or dexamethasone (0.1 to 0.2 mg/kg) for 3 days should be considered in moderate cases if need for oxygen is increasing or there is a rise in inflammatory markers. For severe cases, methylprednisolone 1-2 mg/kg or dexamethasone 0.2-0.4 mg/kg for 5-7 days [12] Adverse effects of steroids are not usually seen in short courses. Chronic use may result in glaucoma, cataract, fluid retention, increased risk of infection etc., |
| Ribavarin | Inhibits RNA dependant RNA polymerase | Haematotoxic | 500 mg for adults, 2-3 times IV. per day (not exceeding 10 days); along with other antivirals like lopinavir/ritonavir |
ADCC: Antibody dependent cell mediated cytotoxicity; NS: Normal saline
Hydroxychloroquine
Inhibitor of Viral Entry and Endocytosis; Immunomodulatory Effects
Chloroquine and HCQ, both are used as drugs for malaria and other conditions like rheumatoid arthritis. Most current efforts to consider these drugs as effective against COVID-19 are based on previous work done demonstrating their antiviral effects specifically on SAR-CoV [40,41]. Yao X et al., demonstrated that both HCQ and chloroquine showed invitro activity against SARS-CoV-2 and HCQ had more potent antiviral activity amongst the two [42]. It is postulated that the activity against the virus may be due to the antiviral and immunomodulatory effects of the drugs, in particular HCQ. In two different Randomised Controlled Trials (RCTs) one by Chen J et al., showed intriguing results for HCQ use in COVID-19 treatment. In one, where moderate COVID-19 cases were given HCQ, no significant benefits were noted over conventional treatment (negative throat swabs in 86.7% HCQ group vs. 93.3% in control group on day seven, median duration from hospitalisation to nucleic acid negativity was four days in HCQ group vs. two days in control group (comparable), body temperature normalisation was also comparable in both groups) [43]. On the other hand, in another RCT by Chen Z et al., where majorly mild cases were considered at entry, reduced body temperature recovery time (0.4 days vs. 1.3 days in control group), reduced cough remission time and greater improvement of pneumonia status (80.6% vs. 54.8% in control group) were seen in the HCQ group [43,44]. Though, majority of patients were young and had mild diseases which may have recovered on its own. Tang W et al., compared HCQ vs standard of care for mild to moderate cases and found that the negative seroconversion rate was not significantly different and also that adverse events occurred in 30% patients in the HCQ arm vs. 8.8% in standard of care arm. Boulware DR et al., evaluated the efficacy of HCQ as a post-exposure prophylaxis agent and evaluated in 821 asymptomatic participants out of which a majority had high risk exposure to a COVID confirmed case. They noted only a 2.4 percentage points difference in the incidence of illness between the two groups (HCQ vs placebo post exposure). Additionally, they noted that the group which received the drug had higher rate of adverse reactions and concluded that this drug may not be effective as a post-exposure prophylaxis agent when given within four days of exposure [45]. Despite the lack of clinical data for safety and efficacy of these drugs, some countries have permitted the usage of these drugs prophylactically and/or for treatment. Recently, a meta-analysis conducted by Singh AK et al., for use of HCQ for the treatment of COVID-19 evaluated three studies that evaluated the Polymerase Chain Reaction (PCR) results post-treatment with HCQ. In their analysis, they found no significant benefits. Additionally, they analysed three studies that reported the mortality outcome in which they found a significant increase in mortality in the HCQ arm [46]. Hernandez AV et al., in a systematic review, concluded that the results were inconclusive for treatment by use of HCQ while there was no data regarding its use for prophylaxis. They evaluated 23 studies for treatment of COVID-19 and noted that mostly the evidence was conflicting to provide any definitive conclusion in favour or against the usage. Majority of RCTs were conducted for HCQ use in COVID-19 cases. They also noted that some patients in several studies showed QT prolongation but again it varied between studies [47]. Majority of RCTs conducted for HCQ do not show significant benefits for use as a treatment modality, but most of these studies are hindered due to multiple factors. Hence, the data about HCQ is not conclusive to advice for or against its usage in COVID-19. More trials may soon produce profound results to guide further management. In India, HCQ has been recommended as a prophylaxis for health care workers and is also being used for management of COVID-19 [48]. A recent study by Indian Council of Medical Research (ICMR) also showed that use of HCQ along with Personal Protective Equipment (PPE) by health care workers could lead to 80% reduction in risk of developing COVID-19 [49].
Hydroxychloroquine with Azithromycin
Azithromycin, a macrolide antibiotic, had been previously shown to be effective against the Zika and the Ebola virus infection [50,51]. Based on these findings, Gautret P et al., conducted a study on 42 patients and added azithromycin along with HCQ for six of their patients and demonstrated a synergistic effect between the two drugs in clearing the viral load and preventing severe respiratory infections [52]. Although, this yielded positive results, the clinical benefit still largely remains unknown and more RCTs are urgently needed [53,54]. To the best of our knowledge, as of when this article was written, there were no published results evaluating the efficacy of azithromycin alone in COVID-19.
Remdesivir
Viral RNA-Dependant RNA Polymerase Inhibitor
Remdesivir showed efficacy against the Ebola virus and since then has been tried for its antiviral effects against different viruses [55-58]. Developed by Gilead sciences, it was shown to have efficacy against the SARS-Cov-2 invitro [59]. It was used on compassionate basis for a patient in the USA and the patient clinically improved which led to a further demand for randomised controlled trials (RCTs) for the drug [60]. As of when this article was written, there were six ongoing studies to evaluate the drug, all of which were phase three clinical trials [61-64]. Beigel JH et al., compared remdesivir to placebo and demonstrated that it was superior to the placebo group [65]. A trial conducted by the National Institute of Allergy and Infectious Diseases demonstrated that 10 days of treatment with remdesivir improved the time to recovery in comparison to placebo [65]. Additionally, a recent study by Goldman JD et al., compared a five day versus a 10-day treatment with remdesivir for multiple end points that included: clinical status at day 14, clinical improvement and time to recovery. They did not find significant differences in the two groups which suggested that a five day course might be effective [66]. Currently, for compassionate use, the manufacturer may also provide the drug as part of an expanded access program only in few countries, in areas where participating in a clinical trial is not an option [67]. Apart from these, as of when this article was written, the drug was in clinical trials phase 3. On 1st June, Gilead Sciences announced the results of the phase 3 SIMPLE trial stating that patients with a five-day course of a drug showed a 65% higher likeliness of clinical improvement on day 11 as compared to standard of care [68]. Remdesivir is also being used in India for management of COVID-19.
Lopinavir/Ritonavir
Inhibitor of proteolysis via 3-chymotrypsin like protease
Anti-retroviral drugs have been in use for HIV for many years now. These drugs had previously showed activity against the SARS-CoV and MERS in-vitro and therefore, were tried against SARS-CoV-2 [69,70]. Although some activity was demonstrated in-vitro by these drugs, a recent study in China by Cao B et al., showed that these drugs did not display any benefits in comparison to standard care and may, in fact, cause additional adverse effects [37]. Similarly, Li Y et al., and Huang M et al., demonstrated that control groups and Chloroquine arm showed better responses, respectively [71,72]. Currently, these drugs are under trials and some studies have suggested that a combination of antivirals may be effective against the virus [73,74].
Convalescent Serum
Preformed Immunoglobulins
After a recommendation for trying convalescent plasma in COVID-19 patients was made in, The Lancet, there has been a surge in research about its efficacy for treatment of critically ill COVID-19 patients [75]. A study reported by Shen C et al., showed that viral load decreased after using plasma and all five patients showed improvement in terms of body temperature normalisation, decreased SOFA (Sequential Organ Failure Assessment) scores, increased PaO2/Fio2 ratios and decreased viral loads and resolution of ARDS [76]. Duan K et al., showed that in a study of 10 participants with severe disease, there was improvement symptomatically and increase in oxyhaemoglobin saturation and seven out of these showed absence of viremia post-therapy [77]. Currently, more than five clinical trials have been proposed to evaluate the efficacy of this method of treatment in different groups of patients based on severity of disease and age groups. It is possible that this might be our best approach right now for treatment and post exposure prophylaxis [78]. Also, in the US, as of 13th April,2020, the Food Drug and Administration (FDA) has provided guidance for investigational use of convalescent plasma in COVID-19 patients [79]. In India, the ICMR has given out letters of intent for phase 2 trials for this therapy [80]. India is currently witnessing a growth in clinical trial submissions to ICMR and even, there have been attempts for off-label usage of plasma therapy in the critically ill patients [81]. Li L et al., reported the first RCT for convalescent serum in China [82]. They evaluated a total of 103 patients and noted clinical improvement in 51.9% patients in the treatment group against 43.1% in the control group. Convalescent plasma treatment was associated with a negative conversion rate of viral PCR at 72 hours in 87.2% of the convalescent plasma group vs. 37.5% of the control group {OR, 11.39 (95% CI, 3.91-33.18); p<0.001}. The authors concluded that addition of convalescent serum to standard of care did not result in statistically significant improvement in time to clinical improvement within 28 days. Though some clinical improvement was seen in severely ill patients, but not in critically ill patients. This first study has similar results to remdesivir and opens the possibilty for trials to evaluate a combination therapy of these two [35]. Another systematic review by Devasenapathy N et al., evaluating the efficacy of convalescent plasma in other severe respiratory viral illnesses provided indirect low quality evidence that it might not be very useful in COVID-19 [83]. There is emerging data about the usage of convalescent plasma and currently it is being used in many countries on an investigational basis but more data is warranted soon. As of now, plasma therapy is being used in India for severe cases and two plasma banks have been created in the capital recently.
Tocilizumab, Sarilumab
IL-6 inhibitors [33]
These drugs majorly act on cytokine release and storm. Xu X et al., showed that in 21 patients, 91% showed improved respiratory function with lower oxygen intake, resolution of lung opacities on Computed Tomography (CT). They also demonstrated improved CRP levels and early resolution of fever with a dose of 400 mg [84]. Guaraldi G et al., showed that use of tocilizumab may reduce the risk of invasive mechanical ventilation or death in COVID pneumonia. They retrospectively evaluated 544 patients, in which 179 were given tocilizumab and 365 had just standard of care. A total of 73 patients died in the standard care group while only 13 died with tocilizumab with a p<0.0001. They also found that 13% patients in drug group had new infections vs 4% in only standard of care group [85]. Another recent study by Biran N et al., included 764 COVID patients in Intensive Care Unit (ICU) and 210 got tocilizumab. They noted a decreased hospital mortality in tocilizimab group (p=0.004) [86]. COVID-19 has been shown to be associated with cytokine storm due to a dysregulated immune system. Tocilizumab is one of the drugs that can potentially be used in this and is available in India. Currently, clinical trials for use of these drugs are ongoing [87,88].
Arbidol
Membrane fusion inhibitor and S protein/ACE2 binding [33].
In 2008, Khamitov RA et al., demonstrated arbidol activity against the SARS virus in-vitro. This has generated interest in it’s use in COVID-19 [89]. A recent study by Wang Z et al., in 69 COVID patients in Wuhan in which they used arbidol (0.4 mg three times a day) in 36 patients and 31 controls, the arbidol group demonstrated improved discharging rate (33% vs 19%) and reduced mortality (0 vs 16%) [90]. The limitation for conclusions was due to small sample size. Although this study gave positive results, a recent systematic review by Huang D et al., of 12 studies showed that arbidol may be associated with a higher negative rate of PCR at day 14 but no other benefits in negative rate at day seven, rate of fever resolution, cough resolution or duration of hospital stay were seen. Thus, they concluded that evidence for using arbidol may not be enough [91].
Camostatmesylate
Camostatmesylate prevents entry into cells via TMPRSS2 [33]. Camostat was shown to inhibit viral entry into cells and thus generated interest [92]. Currently, trials are going on for its use in COVID [93].
Interferons
Most studies that have been performed include interferons as a part of the treatment regimen and not as a solo therapy. A RCT by Davoudi-Monfared E et al., evaluated 42 patients with the IFN-beta1a therapy with a dose of 44 ug/mL three times weekly for two weeks against a control group of 39 patients. The time to a clinical response showed a p-value of 0.95, but interferons showed increased discharge rate and decreased mortality [94]. Likewise, a triple combination phase 2 trial having lopinavir/ritonavir and ribavirin in addition to interferon beta1b by Hung IFN et al., included 86 patients to the intervention group and 41 to the control group. The intervention group had shorter time to negative swab test with a p-value of 0.001 [74]. Currently, there is recommendation against the use of interferons in the US except for its use as a part of a clinical trial [95].
Teicoplanin and Glycopeptides
Blockage of entry of virus by acting on cathepsin L
Teicoplanin is a glycopeptide antibiotic that is used for gram positive bacterial diseases. It was also shown to block entry of SARS-CoV-2 by Zhang J et al., [96]. In a study in Italy, ICU patients with COVID were given teicoplanin. These patients had been given HCQ and tocilizumab and upon admission to ICU teicoplanin was given, 6 mg/kg every 24 hours. Evaluation over 12 days demonstrated improvement in lymphocyte counts, CRP and procalcitonin, but no effect on kidney or liver function was seen. Although this study provided potential benefits, it had a lot of limitations and confusion over use of this drug because severe COVID patients may have Staphylococcus aureus superinfection in which teicoplanin shows improvement. Also, this study only focussed on severe ICU patients and had no comparative group [97].
Oseltamivir
Oseltamivir is a neuraminidase inhibitor that has been used in the treatment of influenza. It was used for patients with COVID-19 in China, although it is very debatable that it might be effective in this disease considering its high specificity [7]. As of when this article was written, oseltamivir had not demonstrated any clinical or invitro efficacy against SARS-CoV-2.
Major Vaccines in Development for COVID-19
As of 3rd September, 2020, there were eight vaccines which entered phase 3 of clinical trials. In all, there are 34 candidate vaccines for clinical evaluation and 142 in preclinical evaluation [31]. From India, there are two indigenous vaccines under human clinical trials. These are by Cadila Healthcare limited and Bharat Biotech. Both of them are in phase 2 of trials. The one being developed by Cadila is a DNA plasmid vaccine while the one by Bharat Biotech is a whole-virion inactivated vaccine [98,99]. Vaccines in phase 3 have been provided in the [Table/Fig-4]. Apart from these, candidate vaccines by Novavax, Anhui ZhifeiLongcom Biopharmaceutical and Curevac are in phase 2 of trials. In China, approval has been given to the candidate vaccine by Sinovac for limited use. The vaccine candidate by Gamaleya is in phase 3 but has been given early approval [100].
Summary of vaccines in phase 3 trials.
| Vaccine developer | Type of vaccine | Number of participants in phase 3 trial |
|---|
| Moderna and NIAID and BARDA | LNP-encapsulated mRNA (mRNA-1273) | 30,000 |
| University of Oxford and AstraZeneca | Simian adenovirus ChAdOx1-S (AZD1222): Non-replicating viral vector (chimp adenovirus) | 30,000 |
| CanSino Biological Inc. and Beijing Institute of Biotechnology | Adenovirus Type 5 Vector (Ad5-CoV): Non replicating viral vector | 40,000 |
| Gamaleya Research Institute | Adeno-based (Gam-COVID-Vac): Non replicating viral vector (Sputnik V) | 40,000 |
| Sinovac | Inactivated (CoronaVac) | 10,490 |
| Wuhan Institute of Biological Products and Sinopharm/Beijing Institute of Biological products | Inactivated | 21,000 |
| Pfizer with FosunPharma and BioNTech | 3LNP-mRNAs (BNT162 a1,b1,b2,c2) | 30,000 |
NIAID: National Institute of Allergy and Infectious Diseases; BARDA: Biomedical Advanced Research and Development Authority; LNP: Lipid Nanoparticle
Miscellaneous Therapeutics
The above mentioned therapies are the ones that are being mostly focussed upon. There are many other drugs that are being repurposed for trials and observational studies that may interact with SARS-CoV-2. Drugs like the Angiotensin Converting Enzyme (ACE) inhibitors that have been used for a long time now for controlling hypertension, have drawn a lot of attention. This is because it was shown that this virus uses ACE2 receptor for its entry into cells [92]. The work in this area has generated mixed results and opinions which show that ACE inhibitors and Angiotensin II Receptor Blockers (ARBs) might be beneficial, harmful or may have no efficacy for use in COVID-19 [101,102]. This conflicting data has raised questions for continued use of these drugs in hypertensive patients. A recent report in The New England Journal of Medicine (NEJM) mentioned that there is huge lack in data that is available and there is an urgent need for recommendations about these drugs [103]. This mixed data has stimulated multiple clinical trials for the same [104,105].
Other drugs and therapies that have been and are being tested include ivermectin, interferons, nitazoxanide, tocilizumab, favipiravir etc. All these need more trials to prove any efficacy in COVID-19 [39,59,106,107]. Currently, favipravir is being used in India by the name of Fabiflu for management of COVID.
Corticosteroids
Decrease pro-inflammatory cytokines and increase anti-inflammatory mediators, activation of histone deacetylase [108]
Corticosteroids have been debated upon since the beginning of this pandemic. Earlier usage of steroids demonstrated worse clinical outcomes and hence, they were not recommended but recently a study by Fadel R et al., showed that short course of methylprednisolone in moderate to severe COVID cases showed better clinical outcomes with reduced hospital stay duration [109]. On 2nd September, 2020, the WHO has given two recommendations for usage of steroids in management of COVID-19. The first is a strong recommendation for usage of systemic steroids in severe and critical COVID-19 which would include complications like ARDS, sepsis and septic shock and severity based on O2 saturations <90% on room air or a respiratory rate >30 per minute in adults or any signs of respiratory distress. The second recommendation is a conditional one against the use of systemic steroids when no such symptoms are present. This means continuing steroids if the patient was already receiving it for some other condition or if the patients develops severe symptoms [110]. This change in use of corticosteroids going from a mixed response and doubts initially, to a recommendation for use in severe and critical COVID-19 came primarily from the announcement of the RECOVERY trial findings [111]. It enrolled a total of 6425 patients out of which 2104 were in the dexamethasone treatment group (6 mg per day for 10 days) while 4321 received the usual care. When the study concluded, in all, 482 (22.9%) patients died in the steroid group while 1110 (25.7%) patients died in the usual care group with a p<0.001. The most encouraging results were that patients getting invasive mechanical ventilation showed a 29.3% mortality rate in dexamethasone group vs. 41.4% mortality rate in the control group at the 28-day point. Also, patients getting oxygen alone had a 23.3% mortality rate vs. a 26.2 rate in control group. This reduction in mortality by one-third and one-fifth, respectively, in these groups were a major turning point for treatment protocols across the world. Although, no benefit was seen due to steroids in the group not receiving respiratory care, this trial generated immense interest [112]. Recently, a meta-analysis was conducted by a WHO working group (REACT). They included seven trials in this meta-analysis (DEXA-COVID19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, Steroids-SARI). The main outcome they focussed on was 28-day all cause mortality in critically ill COVID patients. Finally, there was a total of 1703 patients out of which 678 received corticosteroids and 1025 had received usual care or placebo. They concluded that there were 22 deaths out of 678 in steroid group vs 425 deaths among 1025 in the control group with an OR of 0.66 (95% CI, 0.53-0.82) with a p<0.001. They also reported a secondary outcome of serious adverse events and saw that the RECOVERY trial reported no serious adverse effects while out of the other 6 trials, 64 events were seen in 354 patients and 80 in 342 in control groups. Finally, the reduction in mortality was similar for dexamethasone and hydrocortisone and also similar in lower dose vs. higher dose groups [113]. These trials have changed protocols around the world and now most of them include use of systemic steroids for severe or critical COVID-19.
Conclusion(s)
COVID-19 has suddenly emerged to be a health calamity for the entire world and such a pandemic has not been seen since the Spanish flu back in 1918. The above mentioned therapies are a few that have been tried for either prevention or treatment of COVID-19. A number of trials are still ongoing and results have not come out yet. From the results we have, a few have been positive but not entirely conclusive for clinical use. The major limitation for these therapies has been a lack of RCTs to irrevocably prove their efficacy and safety. Lately, a few medications that were showing benefits in initial trails have failed to be efficacious for COVID-19. As of now, the mainstay for treatment remains supportive care for patients with strict social distancing measures. Hence, the solution for now would be prevention by a triad of strict social distancing in various forms like total lockdowns and self-quarantine, wearing protective gear like face masks and hand washing. The way forward is by trying to flatten the curve while there is ongoing development and research for a vaccine or a drug. This may take some time, but seeing the amount of effort being put in, it may help us see the end of this pandemic soon.
Author contributions: KS, GM and VKK wrote the manuscript. MKD, NK and SG reviewed and edited that manuscript.
Supplemental doc: Supplemental link has been provided with this article which contains-
Summary of published RCTs for hydroxychloroquine, chloroquine, remdesivir and lopinavir/ritonavir and guidelines given by the Indian and Chinese national governments against the ones given by the WHO and CDC to manage COVID-19.
ADCC: Antibody dependent cell mediated cytotoxicity; NS: Normal saline
[1]. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, A novel coronavirus from patients with pneumonia in China, 2019N Engl J Med 2020 382(8):727-33.10.1056/NEJMoa200101731978945 [Google Scholar] [CrossRef] [PubMed]
[2]. Zaki AM, Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA, Isolation of a novel coronavirus from a man with pneumonia in Saudi ArabiaN Engl J Med 2012 367(19):1814-20.10.1056/NEJMoa121172123075143 [Google Scholar] [CrossRef] [PubMed]
[3]. Zhong NS, Zheng BJ, Li YM, Poon Xie ZH, Chan KH, Epidemiology and cause of severe acute respiratory syndrome (SARSGuangdong Peoples Repub China Febr 2003 362(9393):1353-58.10.1016/S0140-6736(03)14630-2 [Google Scholar] [CrossRef]
[4]. Daga M, Kumar N, Aarthi J, Mawari G, Garg S, Rohatgi I, From SARS-CoV to Coronavirus Disease 2019 (COVID-19)-A brief revPeer Reviewed & Open Access Journal 2019 6(4):01-09. [Google Scholar]
[5]. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Early transmission dynamics in Wuhan, China, of novel coronavirus-Infected pneumoniaN Engl J Med 2020 382(13):1199-207.10.1056/NEJMoa200131631995857 [Google Scholar] [CrossRef] [PubMed]
[6]. Liu J, Liao X, Qian S, Yuan J, Wang F, Liu Y, Community transmission of severe acute respiratory syndrome coronavirus 2Emerg Infect Dis 2020 26(6):1320-23.10.3201/eid2606.200239 [Google Scholar] [CrossRef]
[7]. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, ChinaLancet 2020 395(10223):497-506.10.1016/S0140-6736(20)30183-5 [Google Scholar] [CrossRef]
[8]. World Health Organisation. Weekly Operational Update on COVID-19 [Internet]. 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/wou-4-september-2020-approved.pdf?sfvrsn=91215c78_2 [Google Scholar]
[9]. Wu Z, McGoogan JM, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) Outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and preventionJAMA 2020 323(13):1239-42.10.1001/jama.2020.264832091533 [Google Scholar] [CrossRef] [PubMed]
[10]. MoHaF W, Guidance document on appropriate management of suspect/confirmed cases of COVID-19 [Internet]Services DGoH 2020 Available from: https://www.mohfw.gov.in/pdf/FinalGuidanceonMangaementofCovidcasesversion2.pdf [Google Scholar]
[11]. Government of India Ministry of Health & Family Welfare Directorate General of Health Services (EMR Division). Revised Guidelines on Clinical Management of COVID-19 [Internet]. 2020. Available from: https://www.mohfw.gov.in/pdf/RevisedNationalClinicalManagementGuidelineforCOVID1931032020.pdf [Google Scholar]
[12]. Ministry of health and family welfare. CLINICAL MANAGEMENT PROTOCOL: COVID-19 [Internet]. Available from: https://www.mohfw.gov.in/pdf/UpdatedClinicalManagementProtocolforCOVID19dated03072020.pdf. [Accessed 15th July 2020] [Google Scholar]
[13]. World Health Organisation. Home care for patients with suspected or confirmed COVID-19 and management of their contacts [Internet]. 2020. Available from: https://apps.who.int/iris/rest/bitstreams/1292529/retrieve. [Accessed 15th July 2020] [Google Scholar]
[14]. Ministry of health and family welfare. Revised guidelines for Home Isolation of very mild/pre-symptomatic/asymptomatic COVID-19 cases [Internet]. 2020. Available from: https://www.mohfw.gov.in/pdf/RevisedHomeIsolationGuidelines.pdf [Google Scholar]
[15]. CDC. Clinical Care Guidance [Internet]. 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html [Google Scholar]
[16]. Yousefifard M, Zali A, Zarghi A, Madani Neishaboori A, Hosseini M, Safari S, Non-steroidal anti-inflammatory drugs in management of COVID-19; A systematic review on current evidenceInt J Clin Pr 2020 74(9):e1355710.1111/ijcp.13557PMC7267090 [Google Scholar] [CrossRef] [PubMed]
[17]. Organisation WH, Clinical management of COVID-19 [Internet]2020Available from: https://apps.who.int/iris/rest/bitstreams/1278777/retrieve [Google Scholar]
[18]. NIH. Management of Persons with COVID-19 [Internet]. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/ [Google Scholar]
[19]. Elharrar X, Trigui Y, Dols AM, Touchon F, Martinez S, Prud’homme E, Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failureJAMA 2020 323(22):2336-38.10.1001/jama.2020.825532412581 [Google Scholar] [CrossRef] [PubMed]
[20]. Schultz MJ, Dunser MW, Dondorp AM, Adhikari NK, Iyer S, Kwizera A, Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the futureIntensive Care Med 2017 43(5):612-24.10.1007/s00134-017-4750-z28349179 [Google Scholar] [CrossRef] [PubMed]
[21]. NIH. Care of Critically Ill Patients with COVID-19 [Internet]. 2020. Available from: https://www.covid19treatmentguidelines.nih.gov/critical-care/#:~:text=Care%20of%20Critically%20Ill%20Patients%20With%20COVID%2D19&text=The%20Panel%20recommends%20that%20endotracheal,%2C%20if%20possible%20(CIII) [Google Scholar]
[22]. Organisation WH. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020; Available from: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf?sfvrsn=bc7da517_2 [Google Scholar]
[23]. Ranard Lauren S, Fried Justin A, Marwah A, Edmund AD, Givens Raymond C, Deepa K, Approach to acute cardiovascular complications in COVID-19 infectionCirc Heart Fail 2020 13(7):e00722010.1161/CIRCHEARTFAILURE.120.00722032500721 [Google Scholar] [CrossRef] [PubMed]
[24]. Abstract [Internet]. Kidney International Supplements. Elsevier; 2012 [cited 2020 Oct 23]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4089619/ [Google Scholar]
[25]. Ronco C, Reis T, Husain-Syed F, Management of acute kidney injury in patients with COVID-19Lancet Respir Med 2020 8(7):738-42.10.1016/S2213-2600(20)30229-0 [Google Scholar] [CrossRef]
[26]. Coronavirus disease 2019 (COVID-19): Neurologic complications and management of neurologic conditions [Internet]. Available from: https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-neurologic-complications-and-management-of-neurologic-conditions#H2941329361 [Google Scholar]
[27]. Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysisLancet Gastroenterol Hepatol 2020 5(7):667-78.10.1016/S2468-1253(20)30126-6 [Google Scholar] [CrossRef]
[28]. Zhang C, Shi L, Wang FS, Liver injury in COVID-19: Management and challengesLancet Gastroenterol Hepatol 2020 5(5):428-30.10.1016/S2468-1253(20)30057-1 [Google Scholar] [CrossRef]
[29]. NIH. Antithrombotic Therapy in Patients with COVID-19 [Internet]. Available from: https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/antithrombotic-therapy/. [Accessed 15th July 2020] [Google Scholar]
[30]. Institute M. COVID-19 Treatment and Vaccine Tracker 2020 [Internet]. Available from: https://milkeninstitute.org/sites/default/files/2020-04/Covid19%20Tracker%20NEW4-16-20-2.pdf. [Accessed 15th July 2020] [Google Scholar]
[31]. World Health Organisation. Draft landscape of COVID-19 candidate vaccines. 2020. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [Accessed 15th July 2020] [Google Scholar]
[32]. INTERNATIONAL PULMONOLOGIST’S CONSENSUS ON COVID-19 [Internet]. HONDURAS UNAD. INTERNATIONAL PULMONOLOGIST’S CONSENSUS ON COVID-19. 2020. Available from: https://www.unah.edu.hn/dmsdocument/9674-consenso-internacional-de-neumologos-sobre-covid-19-version-ingles [Google Scholar]
[33]. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB, Pharmacologic treatments for Coronavirus Disease 2019 (COVID-19): A reviewJAMA 2020 323(18):1824-36.10.1001/jama.2020.6019PMC7492917 [Google Scholar] [CrossRef] [PubMed]
[34]. Ministry of health and family welfare. Revised advisory on the use of Hydroxychloroquine (HCQ) as prophylaxis for COVID-19 infection [Internet]. Available from: https://www.mohfw.gov.in/pdf [Google Scholar]
[35]. Casadevall A, Pirofski L-A, The convalescent sera option for containing COVID-19J Clin Invest 2020 130(4):1545-48.10.1172/JCI13800332167489 [Google Scholar] [CrossRef] [PubMed]
[36]. Roback JD, Guarner J, Convalescent Plasma to Treat COVID-19: Possibilities and ChallengesJAMA 2020 323(16):1561-62.10.1001/jama.2020.494032219429 [Google Scholar] [CrossRef] [PubMed]
[37]. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, A Trial of lopinavir-ritonavir in adults hospitalised with severe covid-19N Engl J Med 2020 382(19):1787-99.10.1056/NEJMoa200128232187464 [Google Scholar] [CrossRef] [PubMed]
[38]. Cao W, Liu X, Bai T, Fan H, Hong K, Song H, High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with Coronavirus Disease 2019Open Forum Infect Dis 2020 7(3):ofaa10210.1093/ofid/ofaa10232258207 [Google Scholar] [CrossRef] [PubMed]
[39]. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM, The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 invitroAntiviral Res 2020 178:10478710.1016/j.antiviral.2020.10478732251768 [Google Scholar] [CrossRef] [PubMed]
[40]. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M, Invitro inhibition of severe acute respiratory syndrome coronavirus by chloroquineBiochem Biophys Res Commun 2004 323(1):264-68.10.1016/j.bbrc.2004.08.08515351731 [Google Scholar] [CrossRef] [PubMed]
[41]. Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Chloroquine is a potent inhibitor of SARS coronavirus infection and spreadVirol J 2005 2:6910.1186/1743-422X-2-6916115318 [Google Scholar] [CrossRef] [PubMed]
[42]. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Invitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)Clin Infect Dis 2020 71(15):732-39.10.1093/cid/ciaa23732150618 [Google Scholar] [CrossRef] [PubMed]
[43]. Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19J Zhejiang Univ Med Sci 2020 49(2):215-19. [Google Scholar]
[44]. Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, Efficacy of hydroxychloroquine in patients with COVID-19: Results of a randomised clinical trialmedRxiv 2020 3(22):2004075810.1101/2020.03.22.20040758 [Google Scholar] [CrossRef]
[45]. Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, A randomised trial of hydroxychloroquine as postexposure prophylaxis for Covid-19N Engl J Med 2020 383(6):517-25.10.1056/NEJMoa201663832492293 [Google Scholar] [CrossRef] [PubMed]
[46]. Singh AK, Singh A, Singh R, Misra A, Hydroxychloroquine in patients with COVID-19: A systematic review and meta-analysisDiabetes Metab Syndr Clin Res Rev 2020 14(4):589-96.10.1016/j.dsx.2020.05.01732417708 [Google Scholar] [CrossRef] [PubMed]
[47]. Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM, Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: A living systematic reviewAnn Intern Med 2020 173(4):287-96.10.7326/M20-249632459529 [Google Scholar] [CrossRef] [PubMed]
[48]. Ministry of health and family welfare. Revised advisory on the use of Hydroxychloroquine (HCQ) as prophylaxis for COVID-19 infection [Internet]. Available from: https://www.mohfw.gov.in/pdf/evisedadvisoryontheuseofhydroxychloroquineasprophylaxisforSARSCOVID19infection.pdf [Google Scholar]
[49]. Chatterjee P, Anand T, Singh KJ, Rasaily R, Singh R, Das S, Healthcare workers & SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19Indian J Med Res 2020 151(5):459-67.10.4103/ijmr.IJMR_2234_2032611916 [Google Scholar] [CrossRef] [PubMed]
[50]. Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Zika virus cell tropism in the developing human brain and inhibition by azithromycinProc Natl Acad Sci U A 2016 113(50):14408-13.10.1073/pnas.161802911327911847 [Google Scholar] [CrossRef] [PubMed]
[51]. Madrid PB, Panchal RG, Warren TK, Shurtleff AC, Endsley AN, Green CE, Evaluation of Ebola virus inhibitors for drug repurposingACS Infect Dis 2015 1(7):317-26.10.1021/acsinfecdis.5b0003027622822 [Google Scholar] [CrossRef] [PubMed]
[52]. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomised clinical trialInt J Antimicrob Agents 2020 56(1):10594910.1016/j.ijantimicag.2020.10594932205204 [Google Scholar] [CrossRef] [PubMed]
[53]. Lenzer J, Covid-19: US gives emergency approval to hydroxychloroquine despite lack of evidenceBMJ 2020 369:m133510.1136/bmj.m133532238355 [Google Scholar] [CrossRef] [PubMed]
[54]. Molina JM, Delaugerre C, Goff JL, Mela-Lima B, Ponscarme D, Goldwirt L, No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 InfectionMédecine Mal Infect 2020 50(4):38410.1016/j.medmal.2020.03.00632240719 [Google Scholar] [CrossRef] [PubMed]
[55]. Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeysNature 2016 531(7594):381-85.10.1038/nature1718026934220 [Google Scholar] [CrossRef] [PubMed]
[56]. Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and ParamyxovirusesSci Rep 2017 7(43395)10.1038/srep4339528262699 [Google Scholar] [CrossRef] [PubMed]
[57]. Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronavirusesSci Transl Med 2017 9(396)10.1126/scitranslmed.aal365328659436 [Google Scholar] [CrossRef] [PubMed]
[58]. Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Coronavirus susceptibility to the antiviral remdesivir (gs-5734) is mediated by the viral polymerase and the proofreading exoribonucleasemBio 2018 9(2):e00221-18.10.1128/mBio.00221-1829511076 [Google Scholar] [CrossRef] [PubMed]
[59]. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) invitroCell Res 2020 30(3):269-71.10.1038/s41422-020-0282-032020029 [Google Scholar] [CrossRef] [PubMed]
[60]. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, First case of 2019 novel coronavirus in the United StatesN Engl J Med 2020 382(10):929-36.10.1056/NEJMoa200119132004427 [Google Scholar] [CrossRef] [PubMed]
[61]. USNLo M, Severe 2019-nCoV Remdesivir RCT 2020 [Internet]Available from: https://ClinicalTrials.gov/show/NCT04257656. [Accessed 15th July 2020] [Google Scholar]
[62]. USNLo M, Mild/Moderate 2019-nCoV Remdesivir RCT 2020 [Internet]Available from: https://ClinicalTrials.gov/show/NCT04252664. [Accessed 16th July 2020] [Google Scholar]
[63]. USNLo M, Expanded Access Treatment Protocol: Remdesivir (RDV; GS-5734) for the Treatment of SARS-CoV2 (CoV) Infection 2020 [Internet]Available from: https://ClinicalTrials.gov/show/NCT04323761. [Accessed 16th July 2020] [Google Scholar]
[64]. USNLo M, Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734TM) in participants with severe coronavirus disease (COVID-19) 2020 [Internet]Available from: https://ClinicalTrials.gov/show/NCT04292899. [Accessed 16th July 2020] [Google Scholar]
[65]. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Remdesivir for the treatment of Covid-19- Preliminary ReportN Engl J Med 2020 :NEJMoa2007764 [Google Scholar]
[66]. Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Remdesivir for 5 or 10 days in patients with severe Covid-19N Engl J Med 2020 :NEJMoa201530110.1056/NEJMoa201530132459919 [Google Scholar] [CrossRef] [PubMed]
[67]. Sciences G, Emergency Access to Remdesivir Outside of Clinical Trials 2020 [Internet]Available from: https://www.gilead.com/purpose/advancing-global-health/covid-19/emergency-access-to-remdesivir-outside-of-clinical-trials. [Accessed 16th July 2020] [Google Scholar]
[68]. Gilead. Gilead Announces Results From Phase 3 Trial of Remdesivir in Patients With Moderate COVID-19 [Internet]. 2020. Available from: https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/gilead-announces-results-from-phase-3-trial-of-remdesivir-in-patients-with-moderate-covid-19 [Google Scholar]
[69]. Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findingsThorax 2004 59(3):252-56.10.1136/thorax.2003.01265814985565 [Google Scholar] [CrossRef] [PubMed]
[70]. Spanakis N, Tsiodras S, Haagmans BL, Raj VS, Pontikis K, Koutsoukou A, Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimenInt J Antimicrob Agents 2014 44(6):528-32.10.1016/j.ijantimicag.2014.07.02625288266 [Google Scholar] [CrossRef] [PubMed]
[71]. Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y, An exploratory randomised controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalised with mild/moderate COVID-19ELACOI MedRxiv 2020 (2020)10.1101/2020.03.19.20038984 [Google Scholar] [CrossRef]
[72]. Huang M, Tang T, Pang P, Li M, Ma R, Lu J, Treating COVID-19 with ChloroquineJ Mol Cell Biol 2020 12(4):322-25.10.1093/jmcb/mjaa01432236562 [Google Scholar] [CrossRef] [PubMed]
[73]. Choy KT, Yin-Lam Wong A, Kaewpreedee P, Sia SF, Chen D, Yan Hui KP, Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication invitroAntivir Res 2020 178(104786)10.1016/j.antiviral.2020.10478632251767 [Google Scholar] [CrossRef] [PubMed]
[74]. Hung IFN, Lung KC, Tso EYK, Liu R, Chung TWH, Chu MY, Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trialLancet Lond Engl 2020 395(10238):1695-704.10.1016/S0140-6736(20)31042-4 [Google Scholar] [CrossRef]
[75]. Chen L, Xiong J, Bao L, Shi Y, Convalescent plasma as a potential therapy for COVID-19Lancet Infect Dis 2020 20(4):398-400.10.1016/S1473-3099(20)30141-9 [Google Scholar] [CrossRef]
[76]. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Treatment of 5 critically ill patients with COVID-19 with convalescent plasmaJAMA 2020 323(16):1582-89.10.1001/jama.2020.478332219428 [Google Scholar] [CrossRef] [PubMed]
[77]. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Effectiveness of convalescent plasma therapy in severe COVID-19 patientsProc Natl Acad Sci 2020 (202004168)10.1073/pnas.200416811732253318 [Google Scholar] [CrossRef] [PubMed]
[78]. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, Deployment of convalescent plasma for the prevention and treatment of COVID-19J Clin Invest 2020 130(6):2757-65.10.1172/JCI13874532254064 [Google Scholar] [CrossRef] [PubMed]
[79]. FDA. Recommendations for Investigational COVID-19 Convalescent Plasma 2020 [Internet]. Available from: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma [Google Scholar]
[80]. ICMR. Call for Letter of Intent for Participation in: Therapeutic Plasma Exchangein COVID-19: Protocol for a Multi-center, Phase II, Open Label, Randomised Controlled Study. 2020. Available from: https://www.icmr.gov.in/pdf/covid/techdoc/LOI_TPE_12042020.pdf [Accessed 16th July 2020] [Google Scholar]
[81]. Times E. Private hospital in Delhi tries out plasma therapy on two patients 2020 [Internet]. Available from: https://economictimes.indiatimes.com/industry/healthcare/biotech/healthcare/private-hospital-in-delhi-tries-out-plasma-therapy-on-two-patients/articleshow/75171753.cms?from=mdr [Google Scholar]
[82]. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomised clinical trialJAMA 2020 324(5):01-11.10.1001/jama.2020.1004432492084 [Google Scholar] [CrossRef] [PubMed]
[83]. Devasenapathy N, Ye Z, Loeb M, Fang F, Najafabadi BT, Xiao Y, Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: A systematic review and meta-analysisCan Med Assoc J 2020 192(27):E745-55.10.1503/cmaj.20064232444482 [Google Scholar] [CrossRef] [PubMed]
[84]. Xu X, Han M, Li T, Sun W, Wang D, Fu B, Effective treatment of severe COVID-19 patients with tocilizumabProc Natl Acad Sci U S A 2020 117(20):10970-75.10.1073/pnas.200561511732350134 [Google Scholar] [CrossRef] [PubMed]
[85]. Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Tocilizumab in patients with severe COVID-19: A retrospective cohort studyLancet Rheumatol 2020 2(8):e474-84.10.1016/S2665-9913(20)30173-9 [Google Scholar] [CrossRef]
[86]. Biran N, Ip A, Ahn J, Go RC, Wang S, Mathura S, Tocilizumab among patients with COVID-19 in the intensive care unit: A multicentre observational studyLancet Rheumatol [Internet][cited 2020 Sep 2]; Available from: https://doi.org/10.1016/S2665-9913(20)30277-010.1016/S2665-9913(20)30277-0 [Google Scholar] [CrossRef]
[87]. clinicaltrials.gov. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) (TOCIVID-19) [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT04317092 [Google Scholar]
[88]. Roche. Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia [Internet]. Available from: https://www.roche.com/investors/updates/inv-update-2020-07-29.htm. [Accessed 16th July 2020] [Google Scholar]
[89]. Khamitov RA, Loginova SI, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM, Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell culturesVopr Virusol 2008 53(4):09-13. [Google Scholar]
[90]. Wang Z, Yang B, Li Q, Wen L, Zhang R, Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, ChinaClin Infect Dis 2020 71(15):769-77.10.1093/cid/ciaa27232176772 [Google Scholar] [CrossRef] [PubMed]
[91]. Huang D, Yu H, Wang T, Yang H, Yao R, Liang Z, Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): A systematic review and meta-analysisJ Med Virol 2020 :10.1002/jmv.2625610.1002/jmv.2625632617989 [Google Scholar] [CrossRef] [PubMed]
[92]. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitorCell 2020 181(2):271-80.10.1016/j.cell.2020.02.05232142651 [Google Scholar] [CrossRef] [PubMed]
[93]. clinicaltrials.gov. The Impact of Camostat Mesilate on COVID-19 Infection (CamoCO-19) [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT04321096 [Google Scholar]
[94]. Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, A randomised clinical trial of the efficacy and safety of interferon α-1a in treatment of severe COVID-19Antimicrob Agents Chemother 2020 64(9)10.1128/AAC.01061-2032661006 [Google Scholar] [CrossRef] [PubMed]
[95]. NIH. Interferons (Alfa, Beta) [Internet]. Available from: https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/interferons/#:~:text=Interferons%20(Alfa%2C%20Beta)&text=Interferons%20are%20a%20family%20of,and%20in%20vivo%20antiviral%20properties. [Accessed 16th July 2020] [Google Scholar]
[96]. Zhang J, Ma X, Yu F, Liu J, Zou F, Pan T, Teicoplanin potently blocks the cell entry of 2019-nCoVbioRxiv 2020 :2020.02.05.935387 [Google Scholar]
[97]. Ceccarelli G, Alessandri F, d’Ettorre G, Borrazzo C, Spagnolello O, Oliva A, Is teicoplanin a complementary treatment option for COVID-19? The question remainsInt J Antimicrob Agents 2020 562020/05/23 ed(2):106029-29.10.1016/j.ijantimicag.2020.10602932454071 [Google Scholar] [CrossRef] [PubMed]
[98]. CTRI. Novel Corona Virus-2019-nCov vaccine by intradermal route in healthy subjects. [Internet]. Available from: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45306&EncHid=&userName=vaccine. [Accessed 16th July 2020] [Google Scholar]
[99]. clinicaltrials.gov. Whole-Virion Inactivated SARS-CoV-2 Vaccine (BBV152) for COVID-19 in Healthy Volunteers (BBV152) [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT04471519?term=bharat&cond=covid-19&draw=2&rank=1. [Accessed 16th July 2020] [Google Scholar]
[100]. The New York Times. Coronavirus Vaccine Tracker [Internet]. 2020. Available from: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html. [Accessed 17th July 2020] [Google Scholar]
[101]. Bean D, Kraljevic Z, Searle T, Bendayan R, Pickles A, Folarin A, ACE-inhibitors and angiotensin-2 receptor blockers are not associated with severe SARS- COVID19 infection in a multi-site UK acute Hospital TrustmedRxiv 2020 Jan 1 :2020.04.07.2005678810.1101/2020.04.07.20056788 [Google Scholar] [CrossRef]
[102]. Gurwitz D, Angiotensin receptor blockers as tentative SARS-CoV-2 therapeuticsDrug Dev Res 2020 81(5):537-40.10.1002/ddr.2165632129518 [Google Scholar] [CrossRef] [PubMed]
[103]. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD, Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19N Engl J Med 2020 382(17):1653-59.10.1056/NEJMsr200576032227760 [Google Scholar] [CrossRef] [PubMed]
[104]. Clinicaltrialsgov. Losartan for Patients with COVID-19 Requiring Hospitalisation: U.S [Internet]. National Library of Medicine; 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04312009. [Accessed 17th July 2020] [Google Scholar]
[105]. Clinicaltrialsgov. Losartan for Patients with COVID-19 Not Requiring Hospitalisation: U.S [Internet]. National Library of Medicine; 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04311177. [Accessed 17th July 2020] [Google Scholar]
[106]. Totura AL, Bavari S, Broad-spectrum coronavirus antiviral drug discoveryExpert Opin Drug Discov 2019 14(4):397-412.10.1080/17460441.2019.158117130849247 [Google Scholar] [CrossRef] [PubMed]
[107]. Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, Favipiravir versus arbidol for COVID-19: A randomized clinical trialmedRxiv 2020 Jan 1 :2020.03.17.2003743210.1101/2020.03.17.20037432 [Google Scholar] [CrossRef]
[108]. Dexamethasone [Internet]. Available from: https://www.cebm.net/covid-19/dexamethasone/#:~:text=Mechanism%20of%20action&text=Glucocorticoids%20enter%20cells%20and%20bind,responsive%20elements%20and%20transcription%20factors. [Accessed 17th July 2020] [Google Scholar]
[109]. Fadel R, Morrison Austin R, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, Early short course corticosteroids in hospitalised patients with COVID-19Clin Infect Dis 2020 :ciaa60110.1101/2020.05.04.20074609 [Google Scholar] [CrossRef]
[110]. Corticosteroids for COVID-19 [Internet]. 2020. Available from: https://apps.who.int/iris/rest/bitstreams/1299344/retrieve. [Accessed 17th July 2020] [Google Scholar]
[111]. Prescott HC, Rice TW, Corticosteroids in COVID-19 ARDS: Evidence and hope during the pandemicJAMA [Internet] 2020 Sep 2 [cited 2020 Sep 6]; Available from: https://doi.org/10.1001/jama.2020.16747. [Accessed 17th July 2020]10.1001/jama.2020.1674732876693 [Google Scholar] [CrossRef] [PubMed]
[112]. Dexamethasone in hospitalised patients with Covid-19- preliminary reportN Engl J Med [Internet] 2020 Jul 17 [cited 2020 Sep 6]; Available from: https://doi.org/10.1056/NEJMoa2021436. [Accessed 17th July 2020] [Google Scholar]
[113]. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA [Internet]. 2020 Sep 2 [cited 2020 Sep 6]; Available from: https://doi.org/10.1001/jama.2020.1702310.1001/jama.2020.1702332876694 [Google Scholar] [CrossRef] [PubMed]