Despite the developments in gastric cancer, surgical and medical treatments, it still ranks high among the causes of cancer-related death [1]. Radical surgeries, which have been extended in the historical process of gastric cancer treatment, have been replaced by minimally invasive methods today with similar oncological results and applicability [2,3]. Laparoscopic subtotal gastrectomy has became the gold standard treatment method especially in distal localised lesions [4,5]. Despite the increasing success in medical treatments, surgery is still the only chance of cure [6].
Perhaps the most important of many prognostic factors is regional lymph node invasion and survival worsens dramatically in the presence of a positive lymph node [7,8]. Furthermore, the number of positive lymph nodes is one of the most important steps in the correct staging of patients in making adjuvant treatment decisions [9,10]. The best long-term results can be achieved by resection with negative surgical margins and wide lymphadenectomy [11].
Although, it has been shown in a study that lymph node positive patients develop more distant metastases or local recurrence compared to lymph node negative patients, recurrence and metastasis may also develop in lymph node negative patients [12]. In studies conducted, the most important reason for this was found to be possible micrometastases that could not be detected in routine pathological examination and the inability to remove the appropriate number of lymph nodes [13].
In the literature, there is no clear consensus about how many lymph nodes should be removed in early-stage gastric cancers, and studies are mostly focused on the number and rate of positive lymph nodes [14-16]. Therefore, in this study, we aimed to determine the effect of the number of lymph nodes removed in laparoscopic resected, lymph node negative, early stage antral gastric adenocarcinoma on patient prognosis.
Materials and Methods
A retrospective study was conducted in which sixty-two patients who underwent laparoscopic distal gastrectomy and lymph node dissection with the diagnosis of gastric antrum adenocarcinoma in Ankara University Faculty of Medicine Surgical Oncology Clinic between January 2015 and January 2020 and who have pT1N0 pT2N0 and pT3N0 tumour were included. Patients with T4 tumour or positive peritoneal cytology, patients with metastatic disease, patients undergoing emergency or palliative surgery, patients with positive lymph node in pathological examination, and patients whose follow-up results and information were not available were excluded from the study. This study was approved by the Ankara University Faculty of Medicine Ethics Committee (11-33-20).
All patients were diagnosed by preoperative clinical examination, upper GIS endoscopy and biopsies. Compliance of the patients to the operation was determined after staging by preoperative thoraco-abdominal computed tomography. Patient demographic data, operational and pathology results, laboratory values, postoperative follow-up and complications were reviewed retrospectively by data collection assistants consisting of clinical general surgery and surgical oncology specialists over the hospital database. Pathologic stages were determined according to the Tumour Node Metastasis (TNM) classification of the Malignant Tumours (14th edition) [17]. The number of lymph nodes extracted from pathology reports was determined. The patients were divided into two groups according to the number of lymph nodes as below 15 and above 15. While there were 13 (20.9%) patients in the group with below 15 lymph nodes removed, there were 49 (79.1%) patients in the other group with 15 and above lymph nodes removed.
All patients were operated under general anaesthesia and endotracheal intubation. Preoperative 8-hour fasting and routine mechanical bowel cleaning were performed. All routine asepsis and anti-sepsis rules were strictly followed. Preoperative antibiotic prophylaxis was performed with 1 g cefazolin. Pneumo-peritoneum was provided with 13 mm Hg pressure via a 10 mm umblical port, then 5 mm and 10 mm working ports were placed. Distal gastrectomy and partial omentectomy were performed in all patients and lymph node dissection was performed under the guidance of the Japanese Gastric Cancer Treatment Guide [18]. Patients were followed-up with clinical examination and laboratory results for possible complications.
Follow-up: Time uptill 2020 January or mortality considered as overall survival. Time uptill recurrence considered as disease-free survival. Follow-up was performed at third, sixth and ninth months for upto 1 year. After 1 year, follow-up was performed at 6-month intervals, if the patient continued to remain without recurrence or metastasis. Abdomino-pelvic computed tomography were obtained at two times in a year following surgery and adjuvant therapy.
Statistical Analysis
Data were expressed as mean±standard deviation (SD). Chi-square test, Student’s T-test and Mann-Whitney U-test were used for the relationship between numerical and categorical [Table/Fig-1,2]. Kaplan-Meier Survival Curves were used for comparison of overall and disease-free survival rates between the groups and Cox regression analysis with backward elimination method was used to determine the factors affecting overall and disease-free survival. All p-values less than 0.05 were considered statistically significant. These analyses were performed using IBM SPSS statistical version 23.0.
Comparison of clinicopathological variables and postoperative outcomes between two groups of number of lymph node harvested.
| Variables | Total (n=62) | Lymph node <15 (n=13) | Lymph node ≥15 (n=49) | p-value |
|---|
| Age (years) | 59.26±7.31 | 57.15±9.2 | 59.82±6.73 | 0.247 |
| Gender ((male(%)) | 38 (61.3) | 5 (38.5) | 33 (67.3) | 0.058 |
| BMI (Kg/m2) | 25.99±4.39 | 25.63±4.94 | 26.09±4.28 | 0.738 |
| ASA score | | | | |
| 1 | 19 (30.6) | 5 (38.5) | 14 (28.6) | 0.333 |
| 2 | 30 (48.4) | 4 (30.8) | 26 (53.1) | |
| 3 | 13 (21) | 4 (30.8) | 9 (18.4) | |
| T stage | | | | |
| T1 | 9 (14.5) | 0 (0) | 9 (18.4) | 0.109 |
| T2 | 24 (38.7) | 4 (30.8) | 20 (40.8) | |
| T3 | 29 (46.8) | 9 (69.2) | 20 (40.8) | |
| TNM stage* | | | | |
| Stage 1A | 9 (14.5) | 0 (0) | 9 (18.4) | 0.109 |
| Stage1B | 24 (38.7) | 4 (30.8) | 20 (40.8) | |
| Stage 2A | 29 (46.8) | 9 (69.2) | 20 (40.8) | |
| Recurrence/Metastasis | 9 (14.5) | 8 (61.5) | 1 (2) | 0.001 |
| Mortality | 11 (17.7) | 6 (46.2) | 5 (10.2) | 0.007 |
Numerical datas has been presented as mean±standart error. X2 test or Fisher-exact test were used for categorical variables and Student-T test or Mann-whitney U test for numerical variables; p-value<0.05 to be considered significant; BMI: Body mass index; TNM: Tumour-nod-metastasis; *TNM stages has been identified according to 7th edition of UICC TNM classifications of malignant tumours (14); ASA Score: American Society of Anaesthesiologists score; T stage: Tumour stage
Relation between pathological T and TNM stages and recurrence/metastasis.
| T/TNM stage | Recurrence/Metastasis | p-value |
|---|
| None | Exist |
|---|
| T1/Stage 1A | 9 | 0 | 0.287 |
| T2/Stage 1B | 21 | 3 |
| T3/Stage 2A | 23 | 6 |
Fisher-Exact test was used for statistical analysis; TNM stage-: Tumour Node Metastasis stage
Results
The mean age was 59.26±7.31 years and 38 (61%) patients were male. There was no significant difference between the groups in terms of age, gender, BMI, ASA score, T and TNM stage. Of the 9 patients (14.5%) who have developed recurrence or metastasis during follow-up, 8 of them had been seen in the group of less than 15 lymph nodes harvested and the difference was statistically significant (p=0.001). Similarly, the mortality rate was statistically significantly higher in the group of less than 15 lymph nodes (p=0.007) [Table/Fig-1].
No recurrence or metastasis was observed in Stage 1A patients, 3 recurrence or metastasis was observed in Stage 1B and 6 recurrence or metastasis have been seen in Stage 2A patients. Although there was no statistically significant difference (p=0.287), it has been found that patients with more advanced stage disease developed more recurrence or metastasis [Table/Fig-2].
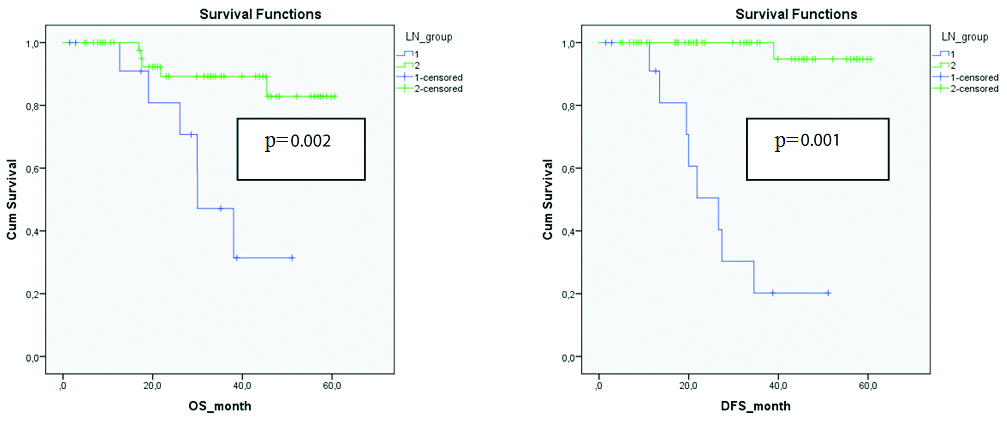
The mean follow-up time was 30.5±17.0 months and the median follow-up time was 29.96±17.0 months. The cumulative survival rate was 97% in the first year and 76.1% in the third year. Mean overall survival in the general patient population was 51.13±2.47 (95% CI: 46.2 ~ 55.9) months, and mean disease-free survival was 52.16±2.48 (95% CI: 47.28 ~ 57, 04) month. The mean overall survival in groups with lymph node removal above and below 15 was 55.07±2.28 (95% CI: 50.60 ~ 59.55) months and 34.80±4.26 (95% CI: 26.43 ~ 43.17) months respectively and the difference was statistically significant (p=0.002). Similarly, mean disease-free survival was 59.43±1.10 (95% CI: 57.26 ~ 61.59) months and 27.85±4.19 (95% CI: 19.63 ~ 36.07), respectively and the difference was statistically significant (p-value=0.001). Kaplan-Meier survival curves comparing overall survival and disease-free survival between the groups are shown in [Table/Fig-3].
Kaplan-Meier survival curves comparing overall and disease-free survival between groups.
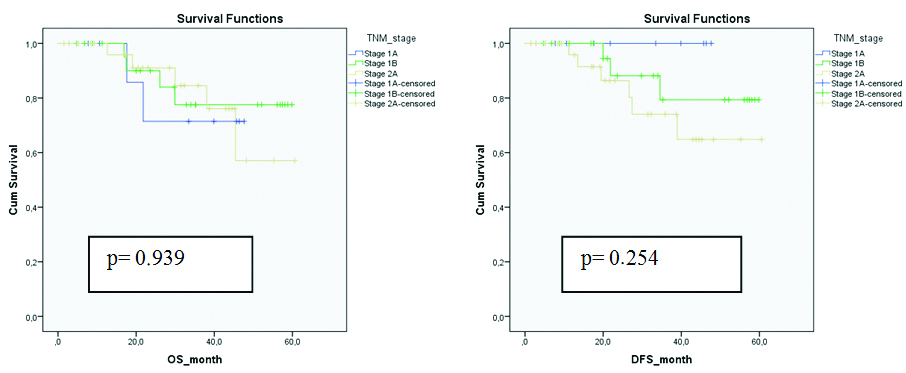
The relationship between overall and disease-free survival of patients with different stages of TNM is shown in [Table/Fig-4]. Although there was no statistically significant difference between the groups in terms of overall and disease-free survival according to TNM stages (p=0.939, p=0.254), it was seen that stage 2A patients with T3 tumours had shorter overall and disease-free survival compared to others.
Kaplan-Meier survival curves comparing overall and disease-free survival in patients with different TNM stages.
Multivariate Cox regression analysis was performed with age over 65, male gender, BMI over 25, ASA score 3, TNM Stage 2A, and 15 and over lymph node removal. As a result of analysis, removal of more than 15 lymph nodes (HR: 12.31, 95% CI: 2.52 ~ 60.19. p=0.002) was found to be independent risk factor of overall survival. Removal of more than 15 lymph nodes (HR: 4.08. 95% CI: 0.78 ~ 21.32. p=0.003) and BMI above 25 kg/m2 (HR: 19.58. 95% CI: 1.14~33.57. p=0.040) were found to be independent risk factors of disease free survival. The results of Cox regression analysis of factors affecting overall and disease-free survival are summarised in [Table/Fig-5].
Cox regression analysis of factors affecting overall and disease-free survival.
| Variable | DFS | OS |
|---|
| HR | %95 CI | p-value | HR | %95 CI | p-value |
|---|
| Age (>65) | 0.33 | 0.02 | 5.04 | 0.428 | 0.44 | 0.08 | 2.27 | 0.332 |
| Gender (male) | 1.13 | 0.22 | 5.89 | 0.877 | 0.99 | 0.25 | 3.91 | 0.998 |
| BMI (25 Kg/m2) | 19.58 | 1.14 | 33.57 | 0.040 | 2.07 | 0.48 | 8.78 | 0.324 |
| ASA score (3) | 0.05 | 0.00 | 1.04 | 0.054 | 0.19 | 0.03 | 1.03 | 0.054 |
| TNM stage (2A) | 0.00 | 0.00 | - | 0.987 | 6.36 | 0.85 | 47.37 | 0.071 |
| Number of lymph node harvested (>15) | 4.08 | 0.78 | 21.32 | 0.003 | 12.31 | 2.52 | 60.19 | 0.002 |
DFS: Disease free survival; OS: Overall survival; HR: Hazard ratio. CI: Confidence interval; BMI: Body mass index; TNM: Tumour-nod-metastasis; p-value <0.05 to be considered significant
Discussion
Adenocancers, which are the most common gastric malignancies, are still among the top causes of cancer-related death, despite a better understanding of tumour biology and countless successes in its treatment [1]. Although many medical treatments have been developed in the historical process, surgical resection and regional lymphadenectomy to be applied at the appropriate stage continue to be the gold standard treatment [6].
Extended radical surgeries have been applied to provide cure for many years, but after extensive randomised studies published in East and West, similar results that could be compared with conventional methods in the treatment of gastric cancer have been achieved [2]. Thus, today, gastric cancer can be treated very effectively with endoscopic and laparoscopic methods [3].
In early gastric cancers, especially in distally located tumours, laparoscopic subtotal gastrectomy has become the gold standard treatment and the feasibility and oncological safety of laparoscopy have been demonstrated. Similarly, the width of lymphadenectomy has begun to be standardised according to the stage and localisation of the tumour. As a result of randomised studies, D2 lymphadenectomy has been widely used in the East except T1 tumours [18,19]. The specimens of two patients underwent distal gastrectomy and omentectomy with D2 lymph node dissection for T2 and T3 antral gastric cancer are shown in [Table/Fig-6,7].
Laparoscopic distal gastrectomy and D2 lymph node dissection for T3 antral gastric adenocarcinoma.

Laparoscopic distal gastrectomy and D2 lymph node dissection for T2 distal antral gastric adenocarcinoma.

Many prognostic factors are known to affect survival outcomes in stomach cancer. Perhaps the most important of these is regional lymph node involvement, and the benefit of a standardised and correctly performed lymphadenectomy to patient survival has been shown in many studies [9,10]. In addition to the survival benefit of the wide standard lymphadenectomy, another important contribution to the oncological results is that it can affect the adjuvant treatment decision by ensuring the correct staging of patients [20]. As the number of positive lymph nodes removed increases, the stage of the disease can also increase. Similarly, the more lymph nodes removed in a case reported as lymph node negative, the more assured the determined phase is.
Although lymph node positive patients have worse long-term survival and higher recurrence rates compared to lymph node negative patients, contrary to general belief, distant metastasis and regional recurrence can be observed in node negative group. The reason for this is the possible micrometastases present in lymph nodes reported as negative in routine pathological examination [13]. In addition, there is no clear information about the possibility of other prognostic factors in the development of recurrence and metastasis in node-negative patients [21].
To date, many different classifications have been developed and modified in the pathological staging of gastric cancer [17]. According to the studies conducted and these pathological classifications, it is recommended to remove a minimum of 15 lymph nodes [22]. In these classifications, while the N stage is determined by the number of positive lymph nodes, the number of lymph nodes removed and the conditions such as the presence of micrometastasis or the size of the micrometastasis- as in breast cancer- are not included in the classification. However, as a result of many randomised studies, it was found that the number of lymph nodes removed was closely related to the prognosis of the patient [20,23]. In a study, the pathological stage deviation was found above 10%, and one of the most important reasons for this was found to be the differences in the number of lymph nodes removed and micrometastases not detected [24]. In another study of patients with lymph node negative gastric cancer, ıt was found that survival and recurrence rates were lower in the patient group with less than 15 lymph nodes harvested compared to the group with more than 15 lymph nodes harvested [23]. In studies conducted by Zhang BY et al., and Baiocchi GL et al., the number of lymph nodes removed was closely related to the patient prognosis [25,26]. In the present study, similar to these studies, the overall survival and disease-free survival rates were found lower in patients who had 15 or more lymph nodes removed regardless of T stage. The possible cause of the relationship between the number of lymph nodes removed and the prognosis can be considered as leaving as few residual diseases as possible.
In the literature, there is no clear data on how many lymph nodes should be removed at which T stage. In the present study, overall and disease-free survival results were found worse in patients with T3 stage compared to T1 and T2 stage patients. Although it is known that the regional lymph node metastasis rate in T1 and T2 stages is quite low, the reason for the worse prognosis despite R0 resection and wide lymphadenectomy in T3 stage can be associated with higher probability of micrometastasis compared to T1 and T2 stages [27]. In the light of all these results, we recommend removing 15 or more lymph nodes in clinical early stage patients in order to reduce recurrence and distant metastasis independent of the T stage, to perform more accurate staging and to improve survival.
Limitation(s)
Since, it is a retrospective study conducted from a single center, possible selection bias and low sample size are the most important limitations of the present study. Patient-dependent and surgeon-related factors may have affected the width of the lymph node dissection, leading the study outcome.
Conclusion(s)
Removing minimum 15 lymph nodes in standard gastrectomy and D2 lymphadenectomy is effective on general and disease-free survival, and we believe that results of the present study are valuable in elucidating this issue. In addition, removing lymph nodes 15 and above can provide more accurate and appropriate staging and affect patients’ decision to be directed towards adjuvant therapy. Our results should be supported by prospective randomised clinical trials in the future.
Numerical datas has been presented as mean±standart error. X2 test or Fisher-exact test were used for categorical variables and Student-T test or Mann-whitney U test for numerical variables; p-value<0.05 to be considered significant; BMI: Body mass index; TNM: Tumour-nod-metastasis; *TNM stages has been identified according to 7th edition of UICC TNM classifications of malignant tumours (14); ASA Score: American Society of Anaesthesiologists score; T stage: Tumour stage
Fisher-Exact test was used for statistical analysis; TNM stage-: Tumour Node Metastasis stage
DFS: Disease free survival; OS: Overall survival; HR: Hazard ratio. CI: Confidence interval; BMI: Body mass index; TNM: Tumour-nod-metastasis; p-value <0.05 to be considered significant