Cirrhosis with portal hypertension is one of the main aetiological factors for hypervolemic hyponatremia. The issue of hyponatremia in liver cirrhosis has received considerable critical attention. In cirrhosis, there is compromise in the effective central blood volume as a result of reflex splanchnic vasodilation, which triggers the compensatory vasoconstrictor and antinatriuretic mechanisms. This results in more free water accumulation than sodium retention, contributing to dilutional hyponatremia [1].
Hyponatremia is relatively common in approximately half of the hospitalised patients with chronic liver disease and in nearly 40% of the outpatients with cirrhosis [2]. The coexistence of hyponatremia seems to have several clinical implications in cirrhosis, being associated with increasing Child Pugh Score, massive ascites, hepatorenal syndrome, hepatic encephalopathy and spontaneous bacterial peritonitis [3,4]. The mortality of patients with hyponatraemia is high in comparison with the patients having normal serum sodium levels [5]. A considerable amount of literature has been published on the importance of sodium levels in the causation of severe liver disease with variable results [2,6].
To date, the data is scarce with regard to the correlation between serum sodium levels and the development of cirrhotic complications. The aim of this study was to explore the relationship between the serum sodium levels and liver cirrhosis with regard to the disease severity and complications.
Materials and Methods
This was a cross-sectional, observational study, conducted between December 2016 and April 2018. Prior to commencing the study, ethical clearance was sought from Institutional Ethics Committee (4073/2016).
The study included all the patients above 18 years of age admitted with the diagnosis of liver cirrhosis. Patients having heart failure, chronic kidney disease and those on thiazide diuretics were excluded. A written, informed consent was obtained prior to the enrollment of the subjects.

Zα-relative deviate (at 95% confidence interval) i.e., 1.96.
p-Prevalence rate of 52%.
e-Allowable error rate of 10%.
N=95
The diagnosis of cirrhosis was established based on clinical features such as jaundice, ascites and signs which included parotid enlargement, spider naevi, gynaecomastia, palmar erythema, dupuytren’s contracture and presence of portosystemic collaterals, liver function test abnormalities such as hyperbilirubinemia, hypoalbuminemia, hyperglobulinemia, reversal of A:G ratio, prolonged prothrombin time, ultrasonographic features of cirrhosis like shrunken liver, nodularity of the surface, edge of the liver, an enlarged caudate and left lobe of the liver [7]. The demographic data of the patients was recorded in a structured proforma. Child Pugh Score was utilised to assess the severity of cirrhosis as class A, B and C [8].
The presence of various complications of cirrhosis which included ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome and variceal bleeding were documented. The presence of hepatic encephalopathy was diagnosed on the basis of speech abnormalities, personality changes, intellectual disorders, and flapping tremors. It was graded as absent, mild or severe [9]. International Ascites Club’s definition was utilised to diagnose hepato-renal syndrome [10].
Spontaneous Bacterial Peritonitis (SBP) is defined as the infection of the ascitic fluid, after excluding perforation of viscera, intraabdominal abscess, acute pancreatitis or cholecystitis [11]. The number of neutrophils in the ascitic fluid must be more than 250 cells/mm and/or bacteriological cultures of the ascitic fluid must be positive showing single organism for the diagnosis of SBP [5].
All the patients underwent complete blood counts, liver function tests, renal function tests, viral markers for hepatitis B and C, abdominal ultrasound and echocardiography.
Serum sodium level was estimated in all the patients at the time of admission. A cut-off of 130 meq/L was considered to define hyponatremia as it is widely accepted definition in cirrhosis [12]. The participants were divided into two groups according to their serum sodium concentration as those with serum sodium ≤130 meql/L and the other group with serum sodium >130 meq/L for comparison of the data. The severity of the liver disease and complications were compared among the two groups.
Statistical Analysis
Collected data was summarised by frequencies and percentages. Tests such as Chi-Square test and Fisher’s-exact test were used appropriately to obtain the significance. A p-value descriptive data were generated for all variables. Odds Ratio (OR) along with Confidence Interval (CI) were calculated for assessing the risk of severity and significant complications of cirrhosis in hyponatremic patients. Receiver Operating Characteristic (ROC) curve analysis was done to find the sodium level cut-off for severity of the disease and complications. All analysis were carried out using SPSS, version 23. Significance level was set at the 5% level.
Results
A total of 95 patients were included in the study. Majority were in the age group of 41-50 years (35.8%) with a mean age of 48.38±11.8 (mean±SD), with the age ranging from 30 to 78 years. There was a male preponderance (91, 95.8%).
The current study found that hyponatremia (≤130 meq/L) was present in 33 patients (34.7%). The difference in the demographic characteristics of the patients between the two groups was not statistically significant. Causative factors for liver cirrhosis included alcoholic liver disease (87, 91.5%), chronic hepatitis B (06 cases, 6.3%) and unknown cause (04 cases, 4.2%). There was no evidence that the aetiology of liver cirrhosis had an influence on the sodium levels in these patients [Table/Fig-1].
Correlation of sodium levels with demographic characteristics and severity of cirrhosis.
| Parameters | | Sodium ≤130 meq/L (N=33) | Sodium >130 meq/L (N=62) | p-value |
|---|
| Age (years) | 30-40 | 9 (27.3%) | 17 (27.4%) | 0.873 |
| 41-50 | 12 (36.4%) | 22 (35.5%) |
| 51-60 | 7 (21.2%) | 10 (16.1%) |
| Above 60 | 5 (15.2%) | 13 (21.0%) |
| Sex | Male | 31 (93.9%) | 60 (96.8%) | 0.512 |
| Female | 2 (6.1%) | 2 (3.2%) |
| Alcohol | Yes | 30 (90.9%) | 57 (91.9%) | 0.864 |
| No | 3 (9.1%) | 5 (8.1%) |
| Hepatitis B | Yes | 2 (6.1%) | 4 (6.5%) | 0.941 |
| No | 31 (93.9%) | 58 (93.5%) |
| Unknown aetiology | | 2 (6.1%) | 2 (3.2%) | 0.512 |
| Child Pugh Score (CPS) |
| A | | 1 (3%) | 1 (1.6%) | 0.029* |
| B | | 3 (9.1%) | 21 (33.9%) |
| C | | 29 (87.9%) | 40 (64.5%) |
*Statistically significant, Fisher’s-exact test
Majority of the patients belonged to Child Pugh C (72.6%). Closer inspection of the [Table/Fig-1] shows that majority of the subjects in the hyponatremic group belonged to Child Pugh C. It is interesting to note that the association of hyponatremia with Child Pugh Score was highly significant with a p-value of 0.029.
An interesting finding was that hepatorenal syndrome and spontaneous bacterial peritonitis occurred more commonly in patients having low sodium levels. The results of this study show that the association of hyponatremia with spontaneous bacterial peritonitis and hepatorenal syndrome was highly significant. However, there was no significant increase in variceal bleeding, hepatic encephalopathy and ascites in patients with low sodium levels [Table/Fig-2].
Correlation of sodium levels with cirrhotic complications.
| Complication | Sodium ≤130 meq/L (N=33) | Sodium >130 meq/L (N=62) | p-value |
|---|
| Ascites | 20 (60.6%) | 38 (61.3%) | 0.948 |
| Hepatic encephalopathy | 13 (39.4%) | 29 (46.8%) | 0.490 |
| Variceal bleed | 10 (30.3%) | 24 (38.7%) | 0.416 |
| Hepatorenal syndrome | 5 (15.2%) | 2 (3.2%) | 0.034* |
| Spontaneous bacterial peritonitis | 11 (33.3%) | 6 (9.7%) | 0.004* |
*Statistically significant, Fisher’s-exact test
The results of the OR for the risk of severity (Child Pugh C) of liver cirrhosis, hepatorenal syndrome and spontaneous bacterial peritonitis in hyponatremic patients is summarised in [Table/Fig-3]. Cirrhotic patients with hyponatremia were at increased risk for severe disease (Child Pugh C, OR 3.987), spontaneous bacterial peritonitis (OR 4.667) and hepatorenal syndrome (OR 5.357) with the risk being maximum for hepatorenal syndrome.
Estimation of Odds Ratio (OR) in hyponatremic patients for risk of severity and complications of cirrhosis.
| Parameter | Odds ratio | 95% CI |
|---|
| Child pugh C | 3.987 | 1.240-12.818 |
| Spontaneous bacterial peritonitis | 4.667 | 1.538-14.164 |
| Hepatorenal syndrome | 5.357 | 0.979-29.327 |
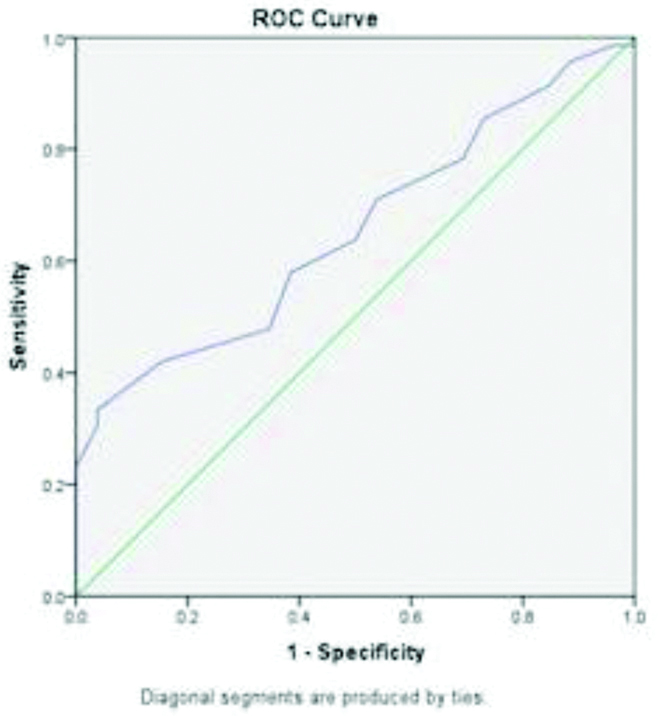
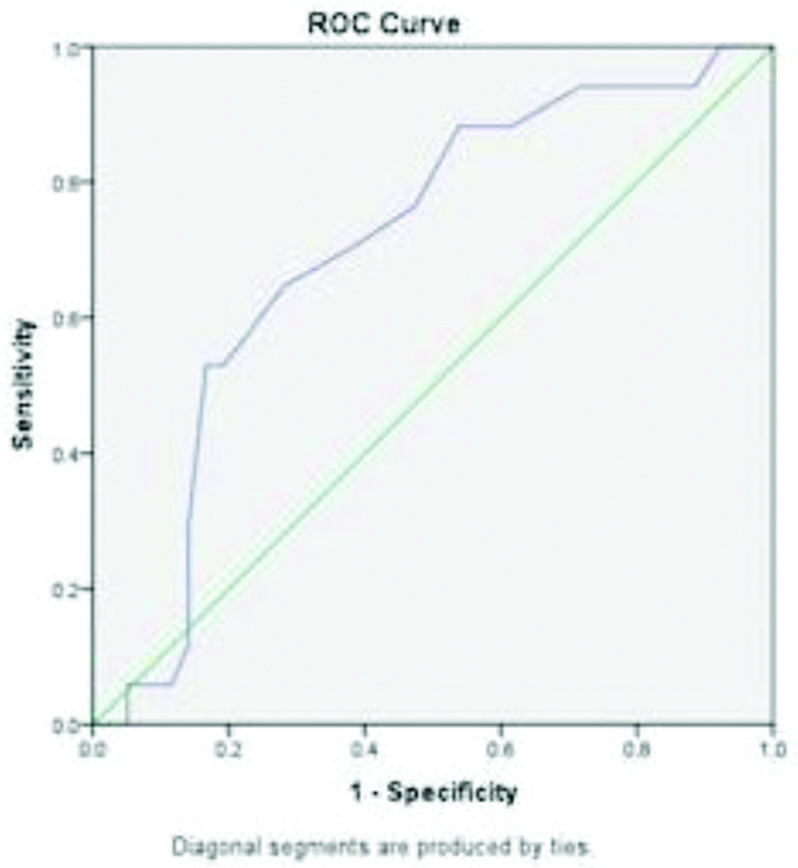
ROC curve analysis showed a sodium level cut-off of 133.5 meq/L for severe liver disease with sensitivity of 63.8% and specificity of 50%. When analysed for complications, a sodium level cut-off was found to be 131.5 meq/L for spontaneous bacterial peritonitis with sensitivity of 70.6% and specificity of 61.5% and 130.5 meq/L for hepatorenal syndrome with sensitivity of 71.4% and specificity of 68.2% [Table/Fig-4,5 and 6].
Receiver Operating Characteristic (ROC) analysis for optimum sodium levels with relation to severity and complications of cirrhosis.
| Parameters | Sodium level cut off (meq/L) | Sensitivity | Specificity | AUC (95% CI) |
|---|
| Child pugh C | 133.5 | 63.8% | 50% | 0.654 (0.540-0.767) |
| Spontaneous Bacterial Peritonitis (SBP) | 131.5 | 70.6% | 61.5% | 0.704 (0.576-0.831) |
| Hepatorenal syndrome | 130.5 | 71.4% | 68.2% | 0.768 (0.634-0.901) |
Area under Curve (AUC) for sodium levels and Child Pugh C.
Area Under Curve (AUC) for sodium levels and spontaneous bacterial peritonitis.
Discussion
Severe neurological symptoms are uncommon in cirrhotic hyponatremia due to the chronicity of the process. In this study, hyponatremia was documented in nearly one third of the subjects. In a multi-center study, the prevalence of hyponatremia at a serum sodium <130 meq/L was 21.6% which is partially comparable to the present study [4]. The findings match those observed in earlier studies [13,14].
One interesting finding was the significant correlation between sodium levels and severity of cirrhosis. This finding is in accordance with previous studies [6,14]. However, a study done in Bangladesh failed to prove any correlation between sodium levels and severity of cirrhosis [15]. The reason for this is not clear but it may be due to the small sample size of the latter study. The observation from the current study may support the hypothesis that hyponatremia in cirrhosis is a marker of severity, being more prevalent in Child Pugh C [12].
One unanticipated finding was the lack of the significant correlation of serum sodium levels with hepatic encephalopathy. This outcome is contrary to the other previous studies [6,14]. It is difficult to explain the inconsistency. However, with a small sample size, caution must be applied, as the finding cannot be extrapolated to all the patients. As per the literature, hyponatremia results in mild cerebral oedema which in turn leads to the formation of glutamine by ammonia. Glutamine accumulation results in swelling and dysfunction of astrocytes, leading to hepatic encephalopathy [3].
Another important finding was the positive correlation between low serum sodium levels and the hepatorenal syndrome. This finding is in concordance with another study done in Pakistan [14]. However, the finding of the present study is contrary to another previous study [6]. Compared to serum creatinine, low sodium levels is a more sensitive marker of impairment in the kidney function [16]. It is believed to be the precursor for the development of hepatorenal syndrome. The possible causation link between hyponatremia and hepatorenal syndrome is due to the more severe circulatory dysfunction in patients with low sodium levels [2].
The results of the study indicate that there was no significant correlation with variceal bleeding and hyponatremia. The findings are consistent with that of Kim JH et al., [6]. The development of varices in cirrhosis is related to the regenerating hepatic nodules compressing the venules and partly due to periportal inflammation or fibrosis which leads to perisinusoidal obstruction. The formation of varices does not depend on the excess of body water. However, there is abundant room for further progress in determining the relationship between hyponatremia and the presence of varices.
The most important clinically relevant finding was the significant association of low sodium levels with spontaneous bacterial peritonitis. This is in line with those of previous studies [4,6,14]. Hyponatremia is associated with more fluid accumulation and thus frequent need for paracentesis [1]. As a result, the chances of spontaneous bacterial peritonitis are higher in these patients. Patients with cirrhosis and spontaneous bacterial peritonitis have contracted effective circulating blood volume which may lead to hepatorenal syndrome and hyponatraemia [17].
The study has enhanced the understanding of possible association of hyponatremia with the severity and complications of liver cirrhosis.
Limitation(s)
The study was limited by the lack of long term follow-up. Considerably, more work needs to be done to determine the clinical implications of hyponatremia in liver cirrhosis.
Conclusion(s)
The evidence from the present study suggests that hyponatremia has a positive correlation with the severity of liver cirrhosis. Its coexistence in cirrhosis poses an increased risk of spontaneous bacterial peritonitis and hepatorenal syndrome. Serum sodium estimation remains a simple, cost effective noninvasive tool recommended in the early course of the disease to predict the severity and complications of cirrhosis.
*Statistically significant, Fisher’s-exact test
*Statistically significant, Fisher’s-exact test