Mullerian Duct Anomalies (MDA) occur due to partial development, incomplete lateral/vertical fusion or absorption of the mullerian ducts. Also known as paramesonephric ducts, a pair of mullerian ducts develop to form the female reproductive tract. The structures formed from the mullerian ducts include uterus, cervix, fallopian tubes and upper one-third of the vagina. The lower one-third of vagina and ovaries have separate embryologic origins [1]. The mullerian duct derivatives of the female reproductive tract have three phases of development: organogenesis, ductal fusion and septal resorption and aberration in these processes result in various MDAs. The lower segment of mullerian ducts fuse to form the uterus, cervix and upper vagina [2]. It has been estimated that 0.16-10% of all women present with MDAs, though the true incidence is unknown [2,3]. Approximately 25% of women with MDAs present with reproductive issues like abortions, intrauterine growth retardation, abnormal foetal lie, etc. Further, approximately 30% of the cases having MDA present with renal anomalies which is due to the embryonic relationship between the mesonephric ducts (which are precursors of renal system) and paramesonephric ducts [2,4,5]. Though Ultrasonography (USG) and Hysterosalpingography (HSG) are used as modalities for initial diagnosis, MRI a non-invasive modality provides the most accurate diagnosis. MRI provides information regarding shape of uterine cavity, presence of septa and external contour of uterine fundus [2]. The newer 3 T MRI has certain advantages over the conventional MRIs. The Signal-to-Noise Ratio (SNR) of conventional MRI is limited and hence, detailed evaluation is not possible. In 3T, there is documented advantage in morphological evaluations. The SNR is also high, which provides better imaging [6].
The differences between the radiological and clinical diagnosis needs to be assessed and rectified. All the previous articles/studies have highlighted the imaging findings in different MDAs [1-2,4]. However, not many studies are conducted to correlate the radiological diagnosis on MRI with clinical diagnosis [3,7]. This study was thus conducted to determine the agreement between the 3T MRI findings of MDAs with clinical diagnosis.
Materials and Methods
The present study was a retrospective observational study which reviewed the MRI abdomen and pelvis images of all females who underwent the imaging in the period of two years: May 2018 till April 2020. Institutional Ethics Committee approval was obtained (protocol number: 2020/028). A total of 1,054 MRIs were screened. All female patients with radiological features of MDA.
Inclusion criteria: Patients without radiological features of MDAs, patients with radiological MDA features but without relevant clinical details from hospital files (one case, which also had no consensus between reporting radiologists) and cases without consensus between the two reporting radiologists (three cases).
Exclusion criteria: Hence, out of 36 cases with MDAs, 33 cases were included for the final diagnosis. MRI was done using 3 T (GE Healthcare, 3 Tesla Signa Pioneer). Sagittal T2W, Sagittal STIR, Coronal T2, Coronal T1W, Axial T2 prop, Axial MERGE, Axial T1FS and Axial T2 were the sequences used for screening of MDAs. The MRI request letter was used to gather information on history and clinical details. Further, the Medical Records Department (MRD) of the institution was requested to provide us with the files of patients diagnosed with MDAs. The details on clinical symptoms, pelvic examination findings, details obtained from imaging modalities other than MRI, findings from surgeries/procedures if performed (laparotomy, laparoscopy, hysteroscopy etc.,) and follow-up were obtained. These examinations were at times conducted in the past/ in some other health facility or after the MRI in our institution. Details on history and pelvic examination findings were available for all the patients. The MRI images were screened for MDAs by two radiologists who were trained in general radiology with two and five years’ experience after postgraduation. At the time of radiological diagnosis, both the radiologists were blinded for clinical diagnosis. Both the radiologists had not undergone sub-specialty training. The consensus of the radiologists for diagnosing the MDAs was checked. The American Society for Reproductive Medicine classification was used for classifying MDAs [8].
Statistical Analysis
The data was entered in Microsoft (MS) excel sheet and was analysed using IBM Statistical Package For Social Sciences (SPSS) (23.0 IBM, New York, USA). The presenting symptoms and types of MDAs were represented as frequencies and percentages. Kappa statistics was used to determine the agreement between the radiological and clinical diagnosis.
Results
A total of 33 patients were included for final diagnosis. Mean age of the study participants was 24.76 (±5.52) years with minimum and maximum ages being 15 years and 36 years, respectively. The prevalence of MDAs in present study was found to be 3.13%. The most common symptom of MDA was repeated miscarriage (36.36%), followed by dysmennorhea (30.30%) and primary amenorrhea (27.27%) [Table/Fig-1]. We observed excellent agreement between the two diagnoses with kappa value 0.80. Agreement was observed among 27 (81.8%) of the cases. [Table/Fig-2] shows the categorisation of the cases according to the American Society for Reproductive Medicine classification and the frequency and proportion of cases that had agreements and disagreements. The disagreements were observed in six cases and the details of MRI diagnosis and clinical diagnosis are summarised in [Table/Fig-3]. The other modalities of imaging like HSG, USG and sonosalpingography were performed for 12, five and one case respectively. The diagnosis on these modalities was included for the clinical diagnosis of the case.
Discussion
The prevalence of MDAs in present study was found to be 3.13% which is similar to the prevalence across the globe, ranging from 0.16-10% [2]. Among 33 cases included for final analysis, 27 cases were found to be having excellent radiological and clinical diagnosis agreement. (κ=0.801). Similar results were obtained in the study conducted by Mueller GC et al., where the investigators observed excellent agreement between the radiological and clinical diagnoses with κ value 0.80. [7]
We diagnosed nine cases with uterine agenesis in present study. Developmental failure of paramesonephric ducts results in uterine or vaginal agenesis/hypoplasia, the types being vaginal, cervical, fundal, tubal or combined. MRI helps in differentiating the uterine agenesis and hypoplasia with best visualisation on sagittal images [9]. [Table/Fig-4] shows fundal agenesis with thin hypoplastic lower uterine segment/cervix (class Ic) and [Table/Fig-5] shows normal uterus with absent ovaries/tubes (classified as class Id). One of the diagnosis of uterine agenesis resulted in disagreement.
Presenting symptoms of the patients diagnosed with MDAs, N=33.*
| Symptoms | n (%) |
|---|
| Primary amenorrhea | 9 (27.27) |
| Repeated miscarriage | 12 (36.36) |
| Dysmenorrhea | 10 (30.30) |
| Irregular menses | 2 (6.06) |
| Vaginal mass | 1 (3.03) |
| Infertility | 7 (21.21) |
| No symptoms | 1 (3.03) |
*Consists of multiple responses
Categorisation of MDAs according to American Society for Reproductive Medicine classification [8] and its agreement and disagreement with clinical diagnosis, N=33.
| American society for reproductive medicine classification | n (%) | Agreement n (%) | Disagreement n (%) |
|---|
| Class I: Hypoplasia/agenesis, n=9 |
| a. Vaginal | 0 (0) | 7 (77.78) | 2 (22.22) |
| b. Cervical | 3 (9.09) |
| c. Fundal | 1 (3.03) |
| d. Tubal | 1 (3.03) |
| e. Complete (Mayer-Rkitansky-Kuster-Hauser syndrome) | 4 (12.12) |
| Class II: Unicornuate, n=9 |
| a. Communicating | 1 (3.03) | 8 (88.89) | 1 (11.11) |
| b. Non-communicating | 3 (9.09) |
| c. With no cavity | 1 (3.03) |
| d. With no horn | 4 (12.12) |
| Class III: Didelphys, n=3 | 3 (9.09) | 3 (100) | 0 (0) |
| Class IV: Bicornuate, n=4 |
| a. Complete | 2 (6.06) | 4 (100) | 0 (0) |
| b. Partial | 2 (6.06) |
| Class V: Septate, n=4 |
| a. Complete septate | 2 (6.06) | 2 (50) | 2 (50) |
| b. Partial septate | 2 (6.06) |
| Class VI: Arcuate, n=4 | 4 (12.12) | 3 (75) | 1 (25) |
| Class VII: DES drug related, n=0 | 0 (0) | 0 (0) | 0 (0) |
Disagreement between the MRI and clinical diagnosis, N=6.
| Serial number | Radiological diagnosis | Clinical diagnosis | Possible reasons for disagreement | Recommendations |
|---|
| 1. | Unicornuate uterus with communicating horn | Unicornuate uterus with non-communicating horn with a thin septum obstructing the non-communicating horn. | No endometrial collection/haematometra, probably due to non-functioning endometrial lining. | Continuity of endometrial lining into the cervix with no haematometra should be the criterion.Thinner sections of MRI with volume imaging.Additional imaging modalities like HSG. |
| 2. | Partial septate uterus | Complete septa | Very thin lower segment septum. | Thinner volume sections of uterus and cervix.Three-dimensional ultrasound.Correlating with clinical history. |
| 3. | Agenesis of uterus, tubes and ovaries | Ovotestis on surgery | Lack of familiarity with signal intensities. | Seeing for position of the organs.Additional imaging modality like HSG. |
| 4. | Septate uterus with incomplete septa | Arcuate uterus | No standard criteria for the diagnosis of arcuate uterus. | Volume rendered MRI with thinner sections. |
| 5. | Arcuate uterus | Submucosal fibroid | Similar signal intensities of submucosal fibrois and myometrium. | Transvaginal sonography. |
| 6. | Haematocolpos with atretic uterus | Infected cyst with adherence to the vaginal wall and uterine agenesis- MRKH | Cyst was simulating the distended vagina/cervix. | HSG/sonosalphingography as an additional imaging modality. |
MRI- Magnetic resonance imaging; HSG- Hysterosalpingography; MRKH- Mayer-Rokitansky-Küster-Hauser
MDA class Ic (a) T2 sagittal and (b) T2 axial images show fundal agenesis with thin hypoplastic lower uterine segment/cervix (yellow arrow).
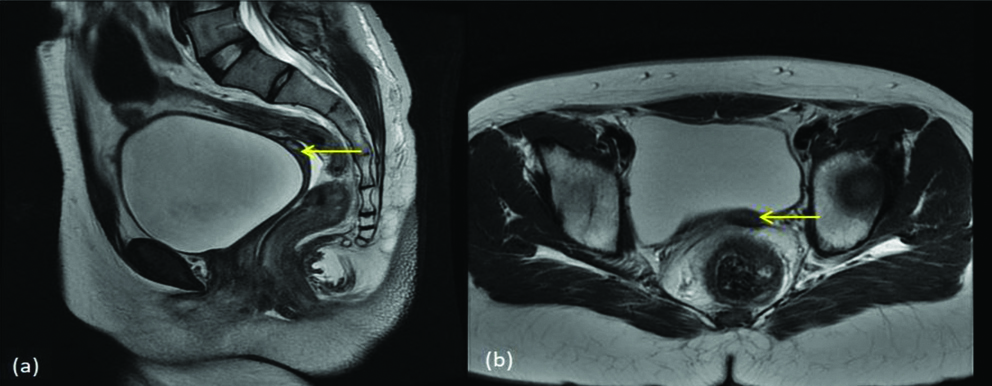
MDA class Id (a) T2 axial (b) STIR coronal and (c) T2 sagittal images show normal uterus (yellow arrow) with absent bilateral ovaries/tubes.
Unicornuate uterus is an asymmetric anomaly where there is non-development or incomplete development of one mullerian duct and the other duct normally develops to form a hemiuterus. These account for about 2%-13% of all MDAs [10,11]. In present study, nine MDAs were diagnosed as unicornuate uteruses. One among them was diagnosed as unicornuate uterus with no cavity (type c) but, it was later clinically diagnosed as unicornuate uterus with communicating horn resulting in disagreement. The [Table/Fig-6] shows two horns of the uterus with the left horn having diffused myometrial hypointensity and haemorrhagic contents within (class IIa). [Table/Fig-7] shows a banana shaped uterus without rudimentary horn (class IId).
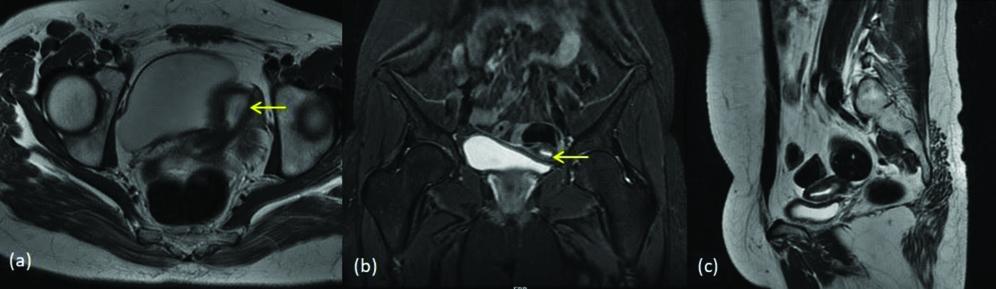
MDA class IIa (a) T2 coronal image (b) T2 axial image shows two horns of the uterus (yellow arrows) with left horn showing diffuse myometrial hypointensity (c) Axial gradient image shows haemorrhagic contents within left horn (blue arrow) indicating obstructed horn.
MDA class IId (a) T2 coronal image (b) T2 sagittal image shows curved and elongated small volume banana shaped uterus (yellow arrow) with its tip to the left side and without rudimentary horn.
Three cases of uterine didelphys were diagnosed in this study and one of the cases had associated renal agenesis. Uterine didelphys results due to complete failure of the fusion of the two mullerian ducts leading to the formation of separate right and left hemiuterus and cervix. This accounts for 5% of the MDAs. Two widely divergent uterine horns and a deep external fundal cleft (>1 cm) are the classical features [1]. [Table/Fig-8] shows the two separate uterine cavities and cervixes with collections indicating an obstruction in vagina. [Table/Fig-9] shows a case of uterine didelphys with renal agenesis.
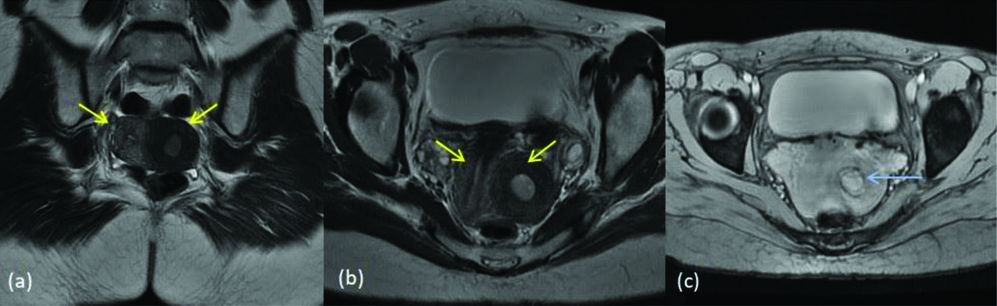
MDA class III- uterine didelphys (a) T2 coronal image (b) T2 axial images show two separate uterine cavities (yellow arrows) (c) T2 STIR axial image shows two separate cervixes (blue arrows). Collections are seen in the endometrial cavities and cervical canal suggesting obstruction in the vagina.
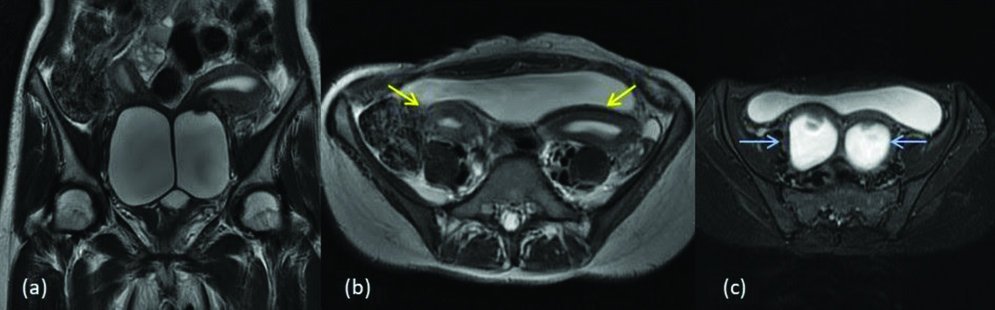
MDA class III- Uterine didelphys with renal agenesis (a) T2 axial (b) T2 coronal images show two separate uterine cavities (yellow arrows) (c) T2 axial image shows two separate cervixes (blue arrows). (d) T2 coronal Image shows right renal agenesis (white arrow).
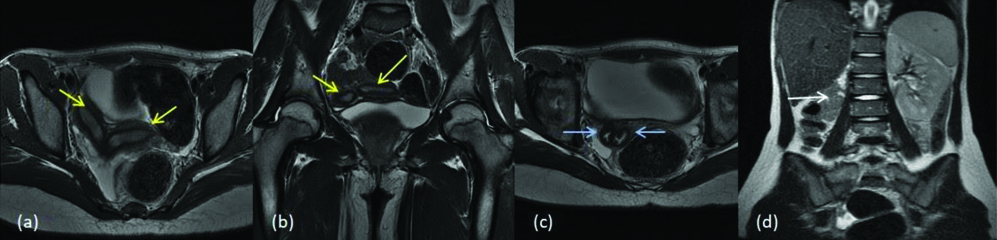
Four cases were diagnosed with bicornuate uterus and among them two had partial and two had complete bicornuate uterus. Approximately, 10% of the MDAs are characterised by incomplete fusion of uterovaginal horns at the level of fundus which results in bicornuate uterus. Lower uterus and cervix are completely fused, which results in the two separate communicating endometrial cavities with a single cervix and vagina and deep external fundal cleft (>1 cm). Based on the length of muscular septum or intervening cleft, bicornuate uterus is classified into bicornuate bicollis or bicornuate unicollis when the cleft extends to the internal cervical os, and when the cleft extends to the external os respectively. Patients with bicornuate uterus have high incidence of spontaneous abortion (28%-35%) and premature birth (14%-23%) [11,12]. [Table/Fig-10] shows a case of bicornuate unicollis with a foetus in one of the horns (class IVb). [Table/Fig-11] shows a case of bicornuate bicollis (class IVa).
MDA class IVb (a) T2 axial (b) T2 axial two separate uterine horns with one cervical canal; central myometrium extends to internal cervical os (yellow arrow) and (c) T2 axial image shows cervix with myometrial septa (blue arrow). Right horn shows the foetus within.
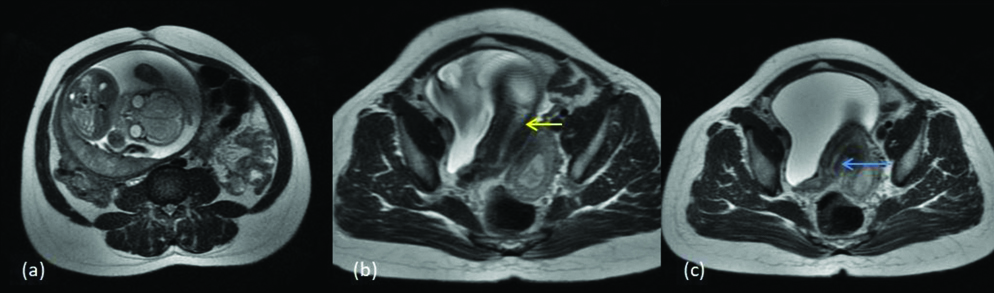
MDA class IVa (a) T2 axial (b) T2 axial two separate uterine horns with two cervical canals; central myometrium extends to external cervical os (yellow arrow) and (c) T2 axial image shows single vagina (blue arrow).
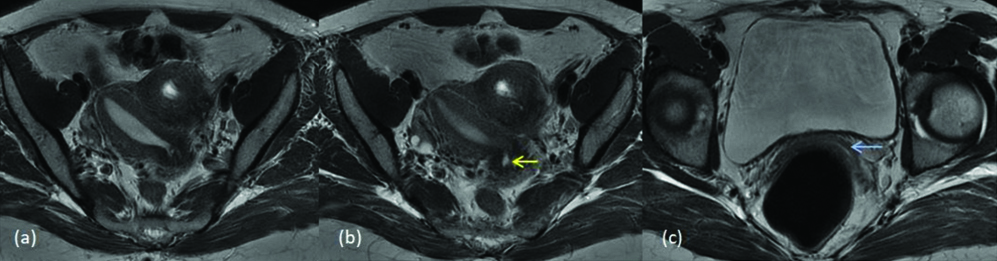
Septate uterus is the most common MDA constituting approximately 55% of the cases [13]. Uterovaginal septum, when fails to resorb after the fusion of paramesonephric ducts, results in a septate uterus. This can be partial or complete failure to resorb. Intercornual distance will be <4 cm and the intercornual angle will be <60° in the septate uterus [9,14]. In present study, four septate uteruses were diagnosed. One was misinterpreted as partial septate uterus and clinical diagnosis of complete septum was given later. Another was diagnosed as incomplete septate uterus, which was later diagnosed as arcuate uterus clinically. [Table/Fig-12] shows flat external uterine contour and complete separation of endometrial canal by septum, not extending into the endocervical canal (class Vb). [Table/Fig-13] shows a mildly convex external fundus with septum extending into the endocervical canal (class Va).
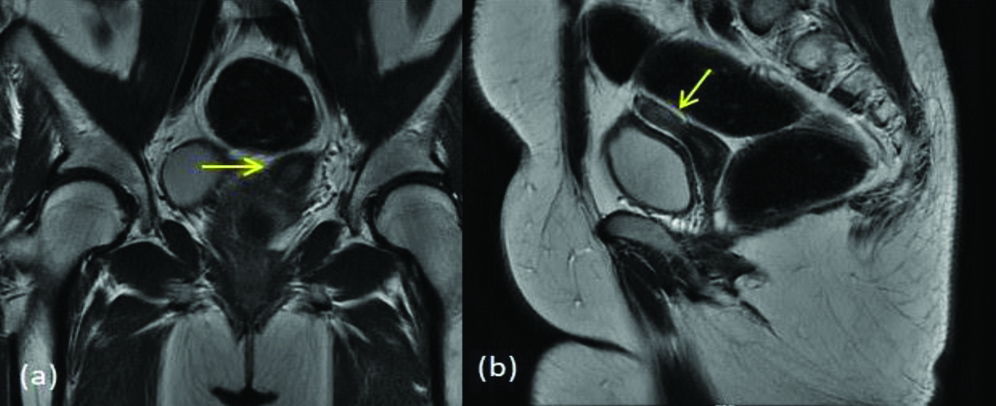
MDA class Vb (a) T2 axial (b) T2 axial (c) T2 coronal (d) T2 axial image shows external uterine fundal contour is flat, endometrial canals are completely separated by septa (yellow arrow) with no extension into endocervical canal (blue arrow).
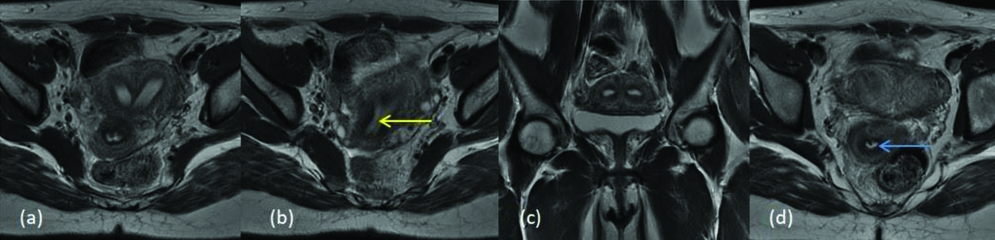
MDA class Va (a) T2 coronal (b) T2 axial (c) T2 axial- shows external uterine fundal contour is mildly convex, endometrial canals are completely separated by septa (yellow arrow) with extension into endocervical canal (blue arrow).
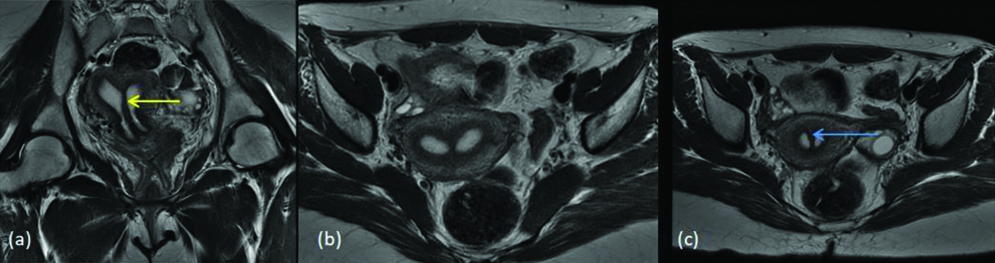
Arcuate uterus results from the near-total resorption of the uterovaginal septum and is characterised by an indentation in the superior aspect of the fundus of the uterus [3]. Four arcuate uteruses were diagnosed and one among them was a misinterpretation which was a submucosal fibroid mimicking arcuate uterus. [Table/Fig-14] shows smooth myometrial fundal indentation, characteristic of arcuate uterus (class VI).
MDA class VI (a) T2 axial (b) T2 STIR (c) T2 coronal images show normal external uterine contour with a small smooth myometrial fundal indentation (yellow arrow).
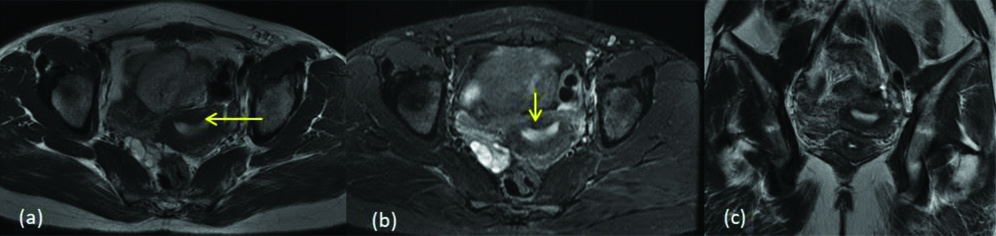
Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome is defined as congenital aplasia of the uterus and upper 2/3rd of vagina [15]. In present study, four cases of MRKH syndrome were diagnosed. These four cases are classified under the ‘type e’ of class I [Table/Fig-15].
MRKH (a) T2 coronal shows normal bilateral ovaries (yellow arrows) with absence of the uterus and upper 2/3rd of vagina (b) T2 sagittal Images (c) STIR axial image shows lower vagina (blue arrow).
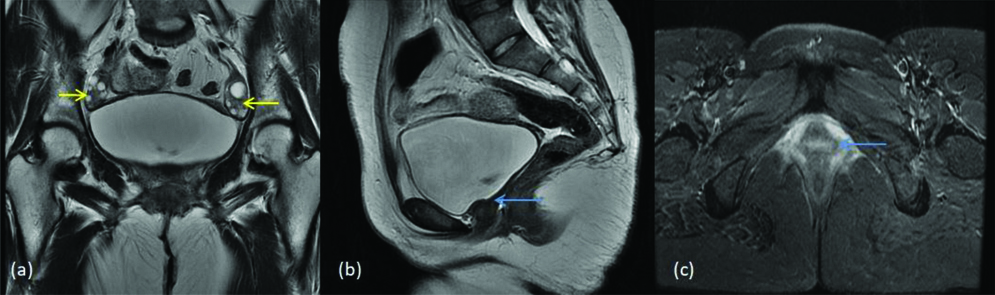
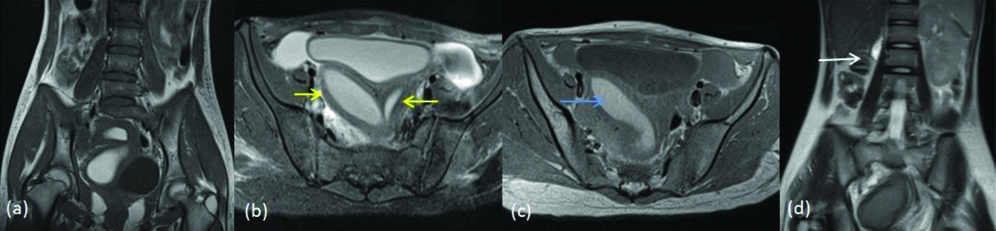
Herlyn Werner Wunderlich syndrome is defined as didelphys uterus with ipsilateral blind hemivagina and renal agenesis. The unilateral renal agenesis is seen in 43% of the cases [16]. In present study, we came across one case of this syndrome and it was diagnosed and classified in class III of American society for reproductive medicine classification [Table/Fig-16].
Herlyn Werner Wunderlich syndrome (a) T2 coronal (b) T2 axial images show two separate uterine cavities (yellow arrows) (c) T1 axial image shows haemorrhage within the right horn of the uterus and right side of cervix (blue arrow) indicating obstruction (d) T2 coronal Image shows absent right kidney. (white arrow).
The drug Diethylstilbestrol (DES), a synthetic non-steroidal estrogen, when exposed in utero can cause MDA. It caused a T-shaped uterus or hypoplasia of the uterus with irregular margins among exposed women [7]. MDAs which are characterised by these findings were not found in present study. Renal agenesis was seen in two of the cases with MDAs. One of them was associated with Herlyn Werner Wunderlich syndrome and the second was with uterine didelphys without any syndromic association. Thus, these findings highlight the fact that renal agenesis is an important finding that needs to be screened and mentioned in cases with MDAs. Causes of disagreement between the radiological and clinical diagnosis were analysed and following observations were made. Image acquisition technique with inappropriate placement of coil and not acquiring thinner volume sections played an important role. Further, certain conditions like arcuate uterus, lack specific imaging criterion which made the diagnosis difficult. Presence of pathological conditions which alter the anatomy like fibroids, endometrial polyps, haemorrhagic cysts can add to misinterpretation. Presence of rare and complex anomalies, unfamiliar pathologies are frequently associated with misinterpretation. Not having a high index of suspicion while interpreting the image and not being an expert in the field of gynaecological imaging can contribute to misinterpretation as well.
3T MRI had very good accuracy to diagnose contour abnormalities of the uterus. It also had excellent accuracy in identifying horns of uterus, duplication abnormalities, abnormalities of the cervix, degree of cervical stenosis etc. The 3T MRI was also able to identify endometrial cavities in duplications of uterine horns, subtle collections within them and also the nature of endometrial collection. It was also very good in identifying horizontal vaginal septum in all the cases of present study. The 3T MRI was good enough to identify ovaries even when they were very small, due to high SNR. However, it observed that 3T MRI was relatively less efficient to analyse the length and thickness of the septum, probably due to its extreme thinness. We opine that, above concern can be addressed by acquiring thinner volume sections of the pelvis in cases with high index of suspicion and also additional imaging modalities like HSG/sonosalpingography needs to be employed.
The strengths of present study are that the data acquisition was done in an efficient manner and with a stringent inclusion criterion. Almost all types of MDAs were captured. The 3T MRI was used for the diagnosis which yielded excellent quality images.
Limitation(s)
Few anomalies can only be picked up using imaging techniques and hence the clinical diagnosis might differ with the radiological diagnosis to a great extent. Also, the sample size of present study was less and the data collection was done in hospital setting. Hence, the result regarding the prevalence of MDAs cannot be generalised.
Conclusion(s)
Excellent agreement was observed between the radiological and clinical diagnosis of MDAs. We conclude that additional imaging modalities should be added whenever necessary, for arriving at an accurate diagnosis. Image acquisition of the uterus and adnexa will also play a major role in visualisation of smaller anatomic details.
*Consists of multiple responses
MRI- Magnetic resonance imaging; HSG- Hysterosalpingography; MRKH- Mayer-Rokitansky-Küster-Hauser