Burkitt’s Lymphoma (BL) is one of the most common paediatric cancers in Sub-Saharan Africa. It is a high-grade lymphoma associated with Epstein Barr Virus (EBV) and malaria infections as co-factors. It may occur as an abdominal tumour with ovarian involvement in a few cases. Previous incidences range between 0.5% to 1.5% of all ovarian neoplasm and are involved in 19% of all adnexal lymphomas. Its occurrence is often among those aged between 6-62 years but very rare among four-year-old girls. This study describes a case of ovarian BL in a four-year-old girl presenting with difficulty in breathing, abdominal pain and distension. The lymphoma had spread to several sites within a short duration. This case represents a great challenge in paediatric oncology management as to when medical or surgical treatment should be considered. Significant laboratory findings were elevated serum Cancer Antigen (CA) 125 and Lactate Dehydrogenase (LDH). Furthermore, flow cytometry, histopathology and immunohistochemistry were confirmed as BL. The child completed chemotherapy and is on remission. Despite its rarity in children, this tumour should be treated aggressively to improve long-term survival.
Case Report
A four-year-old female child admitted with a three-day history of abdominal pain and generalised abdominal distension causing difficulty in breathing. The patient’s mother further complained on behalf of the child of associated malaise and fever for two weeks prior to this admission. The child had a history of herbal ingestion on the second day of abdominal distension and developed difficulty in breathing that worsened over time with the abdominal distension. She had no known food or drug allergies and no history of any blood transfusion. She was a term baby born through Spontaneous Vaginal Delivery (SVD) at home and had no history of any perinatal illness. The child achieved normal milestones, received all required vaccines were exclusively breastfed for six months prior to introduction of mixed feeding. She had no known family history of cancer including gynaecological or colorectal.
She was sick looking, not pale, no cyanosis, not jaundiced and had oxygen flowing via nasal prongs. She had laborious breathing, a body temperature of 38°C respiratory rate of 36 breaths/minute, pulse rate of 150 beats per minute, saturation pressure of oxygen (SpO2) was 90% without external nasal prongs; she weighed 12.5 kilograms and had chest tubes inserted bilaterally due to severe respiratory distress arising from bilateral pleural effusion. Her abdomen was distended, umbilicus everted and moving with respiration. There were no therapeutic scars, hepatomegaly or splenomegaly. She had a suprapubic mass of 10 cm×10 cm that was firm in consistency and non-mobile, tender with no skin changes above it or bruits over the mass. Furthermore, there were no lumbar masses on palpation, adequate bowel sounds heard that were dull in percussion. All other systems were normal.
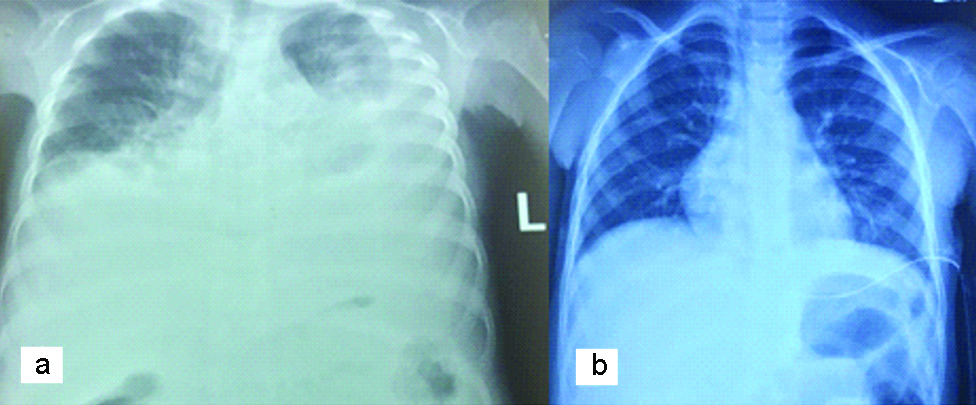
Based on the presentation, the child was suspected to either have an ovarian germ cell tumour, likely dysgerminoma or a lymphoma of the ovary. Chest X-ray showed bilateral pleural effusion [Table/Fig-1].
Chest radiograph showing bilateral pleural effusion. a- Chest X-Ray prior to drainage of pleural effusion. b- Chest X-Ray after drainage of the pleural effusion.
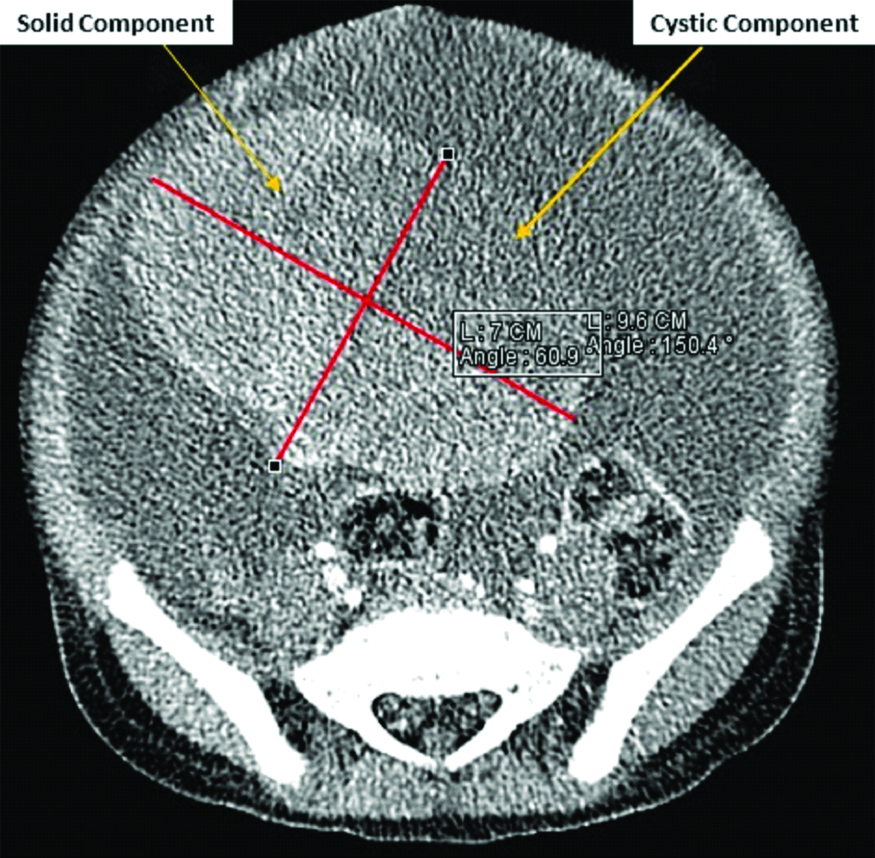
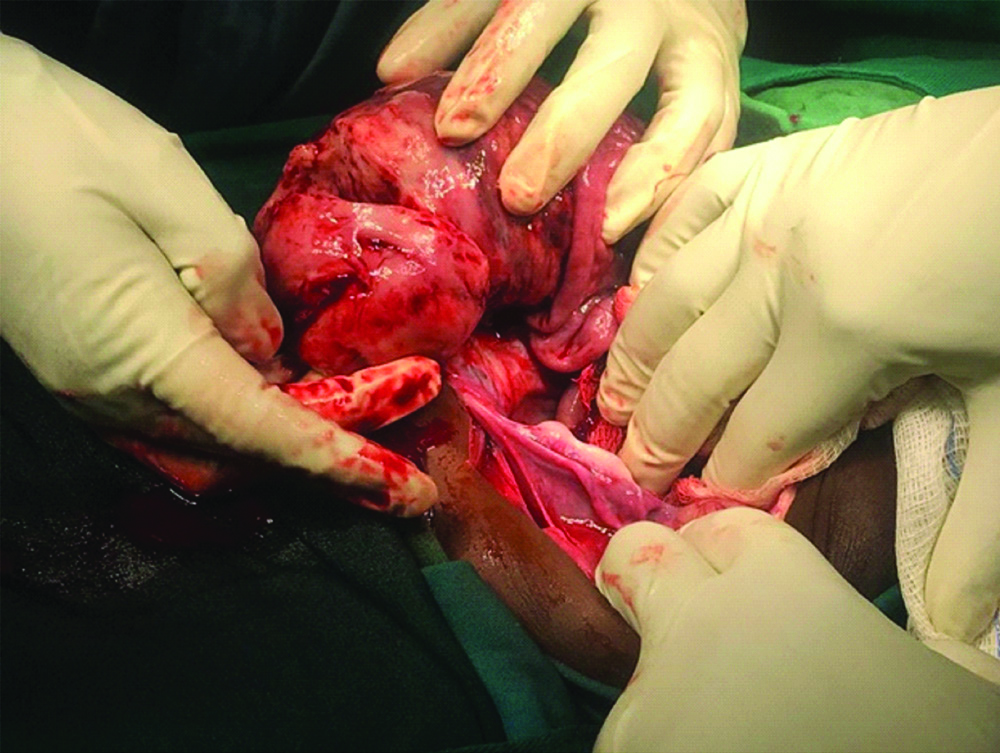
Computed Tomography (CT) scan of the abdomen showed left pelvic mass, had both cystic and solid areas measuring 9.6×7 centimetres [Table/Fig-2]. The mass was likely to be of ovarian origin and had mesenteric infiltration, para-aortic adenopathy, massive ascites and bilateral significant pleural effusion. The echocardiography was normal. Laboratory workup including Complete Blood Count (CBC), liver functions tests and serum tumour markers was ordered. The objective of CBC was to evaluate baseline haemoglobin prior to surgical or medical treatment and assay elevation in leucocyte counts to rule out any associated infections, leukaemic processes or lymphoma. Liver function tests were used to determine the baseline level of liver enzymes prior to treatment and assess liver involvement by the tumour. Serum tumour markers were used to assay for the type of ovarian cancer. The assays showed normal levels of lymphocytes, CA 125, LDH and C-Reactive Protein while albumin and Gamma Glutamyl Transferase (GGT) were low [Table/Fig-3]. Prior to surgery, the provisional diagnosis was ovarian germ cell tumour, highly likely dysgerminoma due to the ovarian mass and the age of the child. A laparotomy was done primarily due to the ovarian mass to confirm the histology and diagnosis. In the theatre, an ovarian mass of 10×10 cm with massive ascites and an omental mass of 2×3 cm was observed [Table/Fig-4].
CT scan abdomen showing predominantly right-sided mixed consistency pelvic lesion.
| Laboratory test | Value | Normal range | Remarks |
|---|
| AFP | 1.48 IU/mL | 0 -5.80 | N |
| CA125 | 1075 U/mL | 0-35 | H |
| CEA | 1.60 ng/mL | 0.000-4.70 | N |
| βhCG | <0.100 mIU/mL | 0.000-1.00 | N |
| LDH | 973 u/L | 60-170 | H |
| HB | 12.2 g/dL | 9.5-14 | N |
| Neutrophils | 10.53*109/L | 2.5-7.5 | H |
| Monocytes | 1.68*109/L | 0.2-0.8 | H |
| Lymphocytes | 3.01*109/L | 1.5-3.5 | N |
| The platelets | 492*109/L | 150-450 | H |
| Calcium | 2.14 mg/dL | 8.5-10.2 | N |
| Magnesium | 0.69 mg/dL | 1.5-2.4 | N |
| Uric acid | 3.34 mg/dL | <5 | N |
| ALP | 102.3 g/dL | 49-290 | N |
| Total bilirubin | 2.8 μmol/L | 5.1-17 | N |
| ALT | 6 u/L | 5-45 u/L | N |
| Total protein | 6.0 g/dl | 6-8 | N |
| Coagulation profile | Normal ranges | | N |
| HIV, HCV and HbSAg | Negative | - | N |
| GGT | 4.7 u/L | 5-27U/L | L |
| Albumin | 30 g/L | 35-55 | L |
| CRP | 76.61 mg/L | 0-10 | H |
L: Low; N: Normal; H: High; AFP: Alpha feto protein; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; CRP: C reactive protein; CEA: Carcinoembryonic antigen; hCG: Human chorionic gonadotropin; LDH: Lactate dehydrogenase; HB: Haemoglobin; HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; HBsAg: Hepatitis B surface antigen; GGT: Gamma glutamyl transferase
Ovarian mass during explorative laparotomy. An ovarian mass of 10×10 cm with massive ascites and an omental mass of 2×3 cm.
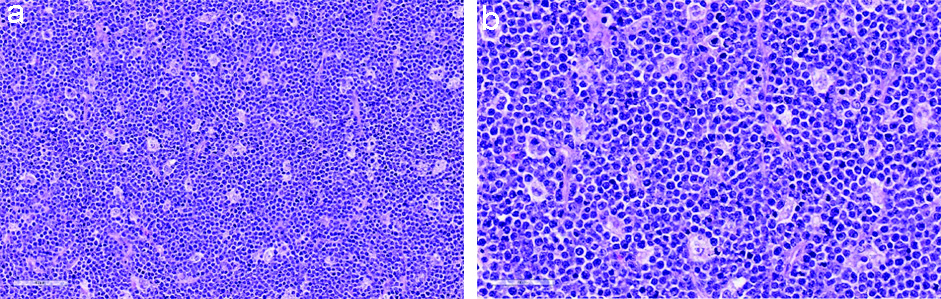
On explorative laparotomy, the uterus could not be visualised and there was no palpable para-aortic lymphadenopathy. A unilateral salpingo-oophorectomy was done. Samples for flow cytometry analysis from the ovarian tissue were collected during surgery as part of the laboratory workup. This showed remarkable monoclonal B-cell population positive for CD19, CD10 and lambda clonality that was suggestive of Burkitt’s lymphoma [Table/Fig-5]. The gross histopathological findings showed large friable mass of 100 mm in diameter with areas of necrosis on cut sections. The tumour was composed of a diffuse infiltrate of small to intermediate cells with irregular nuclei, coarse chromatin pattern, inconspicuous nucleoli and scant cytoplasm. Numerous tingible body macrophages were present creating a ‘starry sky’ appearance. Mitotic activity was brisk [Table/Fig-6]. On immunohistochemistry, the tumour cells showed a strong, membranous CD 20 is positive at 99% and had a remarkably high (>99%) Ki-67 proliferation index. This was concluded as morphological features of Burkitt’s lymphoma [Table/Fig-7].
| Panel | Percentage score (%) | Blood cells | Percentage score (%) |
|---|
| T-cell panel | Granulocytes | 2 |
| sCD3 | 2 | Monocytes | <1 |
| cyCD3 | - | Lymphocytes | 2 |
| CD4 | 1 | Erythrocytes | <1 |
| CD8 | 1 | CD45-Dim | 95 |
| CD5 | 2 | | |
| B-cell panel | Myeloid panel |
| CD19 | 84 | CD117 | - |
| CD20 | 99 | CD13 | - |
| CD10 | 96 | CD33 | - |
| kappa | 1 | MPO | - |
| lambda | 69 | CD14 | - |
| cyCD79a | - | CD15 | - |
| Others | |
| HLA-DR | - |
| CD34 | - |
| CD23 | - |
| TDT | - |
| Blasts | - |
CD: Cluster of differentiation; HLA-DR: Human leucocyte antigen-DR isotype; TDT: Terminal deoxynucleotidyl transferase; MPO: Myeloperoxidase
Histopathology images using Haematoxylin & Eosin staining.
a: Small round blue cells with dispersed tingible body macrophages creating a starry sky appearance (H&Ex20); b: The tumour cells are small to intermediate with scant cytoplasm (H&E)x40)
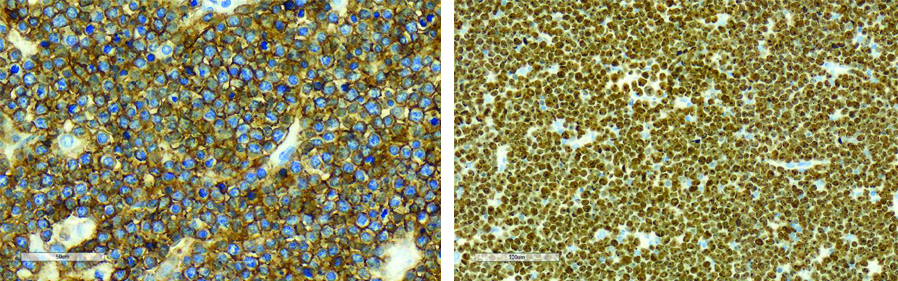
Immunohistochemistry images (using CD20 and Ki67 antibodies).
a: CD 20 Strong membranous staining (x40); b: Ki 67 (x20) Very high proliferative index (>99%)
The patient was initiated on prephase chemotherapy consisting of cyclophosphamide, vincristine, methotrexate, hydrocortisone, and prednisolone followed by hydration over one week. Hyperhydration was done on the seventh day followed by the first course of chemotherapy that was like the prephase regimen alongside folinic acid. Seven more chemotherapy cycles were administered every four weeks that alternated doxorubicin with cyclophosphamide alongside the standard regimen. After the eighth course of chemotherapy, the patient was followed-up monthly for three months. Currently the patient is undergoing a three-monthly treatment follow-up.
Discussion
Ovarian cancer is the second most common cause of cancers among women in Sub-Saharan Africa; and the second most common cause of mortality among women with gynaecologic tumours in the United States of America after uterine cancers [1]. Although paediatric ovarian tumours are rare, an annual incidence of 2.6 per 100,000 girls has been reported from retrospective studies [2]. Most of these paediatric tumours are benign while nearly one third are malignant and these account for about 3% of all childhood malignancies [3]. Ovarian tumours are either germ cell, epithelial, sex-cord stromal or metastatic tumours from other sites. A rare occurrence is the neoplasms of ovarian soft tissues (lymphomas) or non-neoplastic processes.
Ovarian Burkitt’s lymphoma can be classified as either primary or secondary ovarian lesions [4]. Paediatric Burkitt’s lymphoma is prevalent in Western Kenya [5,6] which is a malaria holoendemic region. Patients presenting with ovarian tumours could complain of lower abdominal pain or a palpable mass [3]. Presentation of ovarian Burkitt’s lymphoma is varied and usually presents as rapidly developing symptoms. The diagnosis of these tumours is often difficult and can be delayed due to non-specific symptoms at presentation and on imaging [7]. The definitive diagnosis is from biopsy specimen, followed by supportive and chemotherapy treatment with a good prognosis expected [8].
Primary ovarian Burkitt’s lymphoma is a rare condition due to the absence of lymphoid tissue in the ovary [7]. It is mainly the manifestation of the sporadic Burkitt’s lymphoma subtype, which often presents as an abdominal tumour [4]. Previous reported incidences range between 0.5% to 1.5% of all ovarian neoplasm and are involved in 19% of all adnexal lymphomas [9]. However, there are few reported ovarian tumour cases in the paediatric population [7]. Although germ cell tumours are often reported, they are not commonly seen in healthcare facilities within the developing economies. This makes it difficult to be detected in resource limited healthcare settings such as those in East Africa.
The presentation of ovarian Burkitt’s lymphoma is multifaceted, atypical and lacks a standardised screening tool further complicating diagnosis. The least invasive procedure should be used in the sample collection for cytologic and pathologic evaluation of the involved tissue biopsy. There is also need to expedite the staging workup because Burkitt’s tumour grows rapidly, causing life-threatening complication [10]. Histology of biopsy specimen is the currently used for its diagnosis in Kenya [11]. This is made by obtaining tumour cells for histopathology, immunohistochemistry, and flow cytometry. In this case-report, laparotomy was done primarily due to the ovarian mass and to confirm the histology. Flow cytometry and immunohistochemistry tests were done on the ovarian tissue sample obtained during surgery.
To manage ovarian Burkitt’s lymphoma, surgeons remove the masses revealed by imaging techniques to confirm the diagnosis through histopathology [12]. A typical morphological spectrum shows high expression of B-cell markers: CD 20, CD 22, CD 19, and CD 10 as well as negative stain against cytokeratin and T-cell markers assayed through flow cytometry. We report elevated CD19 and CD10 and lambda on the B-cell panel while low proportions of sCD3, CD4, CD8 and CD5 on the T-cell panel on flow cytometry and expression of Ki67 and CD20 on immunohistochemistry. Clinical biochemistry techniques assaying for elevated CA 125 levels have also been adopted in many clinical settings. The child had elevated CA 125 and LDH serum levels.
Imaging studies are helpful in evaluating the extent of disease and are used in assessing sites of relapse [7]. The child’s CT scan of the abdomen showed a left pelvic mass with both cystic and solid areas that were likely to be of ovarian origin with mesenteric infiltration. Complete staging is helpful; however, treatment should not be delayed while extensive tests are performed.
Lymphomas are associated with aberrant genitalia complicating follow-up and quality of life [13,14]. On the other hand, the definitive treatment for ovarian lymphomas is by chemotherapy [15]. It is the mainstay of treatment of Burkitt’s lymphoma hence, the role of surgery or radiation therapy is limited in the treatment [10]. CODOX-M/IVAC regimen (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, Etoposide, high-dose cytarabine) is used. These tumours are radiosensitive but due to the severity of side effects and the fact that disease is usually not localised chemotherapy remains superior choice. Chemotherapy improves overall 5-year patient survival rate by 80% in primary ovarian lymphoma and 33% in secondary ovarian lymphoma [9]. The child has completed the CODOX-M/IVAC regimen protocol, is under remission and on three-monthly follow-up.
It is also critical to closely monitor serum chemistries, because of tumour lysis syndrome and uric acid nephropathy. Aggressive hydration and prophylactic allopurinol are used to prevent tumour lysis syndrome and its complications, while intravenous antibiotics should be administered for neutropenic fevers. Growth factors such as Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) or Granulocyte Colony-Stimulating Factor (G-CSF) should be administered to decrease the duration of neutropenia [16]. Red blood cells and platelets transfusions are indicated for anaemia and thrombocytopenia. All blood products should ideally be leukodepleted and irradiated so as to reduce fevers associated with non-haemolytic transfusion reactions [17].
Conclusion(s)
The case reported in this study presented with a distended abdomen and difficulty in breathing resulting from a metastasis of disease. This poses a great challenge as there is no screening tool or test for ovarian lymphoma in children. Due to rapid growth of these tumours, the clinical picture can misguide treatment options. It is imperative for clinicians to have a high index of suspicion in young children who present with abdominal tumours or masses. The utility of flow cytometry could be beneficial before invasive surgery. Explorative laparotomy with oophorectomy should be used to obtain a biopsy that can be used to establish the histologic type before chemotherapy.
L: Low; N: Normal; H: High; AFP: Alpha feto protein; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; CRP: C reactive protein; CEA: Carcinoembryonic antigen; hCG: Human chorionic gonadotropin; LDH: Lactate dehydrogenase; HB: Haemoglobin; HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; HBsAg: Hepatitis B surface antigen; GGT: Gamma glutamyl transferase
CD: Cluster of differentiation; HLA-DR: Human leucocyte antigen-DR isotype; TDT: Terminal deoxynucleotidyl transferase; MPO: Myeloperoxidase
[1]. Brookfield KF, Cheung MC, Koniaris LG, Sola JE, Fischer AC, A population-based analysis of 1037 malignant ovarian tumours in the pediatric populationJ Surg Res 2009 156(1):45-49.10.1016/j.jss.2009.03.06919592022 [Google Scholar] [CrossRef] [PubMed]
[2]. Anthony EY, Caserta MP, Singh J, Chen MYM, Adnexal masses in female pediatric patientsAm J Roentgenol 2012 198(5):426-31.10.2214/AJR.11.792022528923 [Google Scholar] [CrossRef] [PubMed]
[3]. Hanafy AK, Mujtaba B, Yedururi S, Jensen CT, Sanchez R, Austin MT, Imaging in pediatric ovarian tumoursAbdom Radiol 2020 45(2):520-36.10.1007/s00261-019-02316-531745573 [Google Scholar] [CrossRef] [PubMed]
[4]. Epelman M, Chikwava KR, Chauvin N, Servaes S, Imaging of pediatric ovarian neoplasmsPediatr Radiol 2011 41(9):1085-99.10.1007/s00247-011-2128-x21567140 [Google Scholar] [CrossRef] [PubMed]
[5]. Sumba PO, Kabiru EW, Namuyenga E, Fiore N, Otieno RO, Moormann AM, Microgeographic variations in Burkitt’s lymphoma incidence correlate with differences in malnutrition, malaria and Epstein-Barr virusBr J Cancer 2010 103:1736-41.10.1038/sj.bjc.660594721102592 [Google Scholar] [CrossRef] [PubMed]
[6]. Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R, Spatial distribution of Burkitt’s lymphoma in Kenya and association with malaria riskTrop Med Int Heal 2007 12(8):936-43.10.1111/j.1365-3156.2007.01875.x17697088 [Google Scholar] [CrossRef] [PubMed]
[7]. Ruttenstock EM, Saxena AK, Schwinger W, Sorantin E, Hoellwarth ME, Pediatric ovarian tumours- Dilemmas in diagnosis and managementEur J Pediatr Surg 2010 20(2):116-20.10.1055/s-0029-124619820112185 [Google Scholar] [CrossRef] [PubMed]
[8]. Taskinen S, Fagerholm R, Lohi J, Taskinen M, Pediatric ovarian neoplastic tumours: Incidence, age at presentation, tumour markers and outcomeActa Obstet Gynecol Scand 2015 94(4):425-29.10.1111/aogs.1259825640522 [Google Scholar] [CrossRef] [PubMed]
[9]. Al-Maghrabi H, Meliti A, Primary bilateral ovarian burkitt lymphoma; A rare issue in gynecologic oncologyJ Surg Case Reports 2018 5:01-03.10.1093/jscr/rjy11329876052 [Google Scholar] [CrossRef] [PubMed]
[10]. Morowitz M, Huff D, Von Allmen D, Cass D, Benjamin B, Epithelial ovarian tumours in children: A retrospective analysisJ Pediatr Surg 2003 38(3):331-35.10.1053/jpsu.2003.5010312632344 [Google Scholar] [CrossRef] [PubMed]
[11]. Bacalbasa N, Stoica C, Popa I, Mirea G, Balescu I, Endometrial carcinoma associated with ovarian granulosa cell tumours-A case reportAnticancer Res 2015 35(10):5547-50. [Google Scholar]
[12]. Stepniak A, Czuczwar P, Szkodziak P, Wozniakowska E, Wozniak S, Paszkowski T, Primary ovarian burkitt’s lymphoma: A rare oncological problem in gynaecology: A review of literatureArch Gynecol Obstet 2017 296(4):653-60.10.1007/s00404-017-4478-628770352 [Google Scholar] [CrossRef] [PubMed]
[13]. Rubio-Gonzalez B, Zain J, Rosen ST, Querfeld C, Clinical manifestations and pathogenesis of cutaneous lymphomas: Current status and future directionsBr J Haematol 2017 176(1):16-36.10.1111/bjh.1440227782301 [Google Scholar] [CrossRef] [PubMed]
[14]. Gru AA, Dehner LP, Cutaneous hematolymphoid and histiocytic proliferations in childrenPediatr Dev Pathol 2018 21(2):208-51.10.1177/109352661775094729607757 [Google Scholar] [CrossRef] [PubMed]
[15]. Billmire DF, Childhood lymphomaSurg Child Tumours 2016 :38310.1007/978-3-662-48590-3_21 [Google Scholar] [CrossRef]
[16]. Sampattavanich S, Steiert B, Kramer BA, Gyori BM, Albeck JG, Sorger PK, Encoding growth factor identity in the temporal dynamics of FOXO3 under the combinatorial control of ERK and AKT kinasesCell Syst 2018 6(6):664-78.e9.10.1016/j.cels.2018.05.00429886111 [Google Scholar] [CrossRef] [PubMed]
[17]. Sharma RR, Marwaha N, Leukoreduced blood components: Advantages and strategies for its implementation in developing countriesAsian Journal of Transfusion Science 2010 4:03-08.10.4103/0973-6247.5938420376259 [Google Scholar] [CrossRef] [PubMed]