Role of laboratory in clinical decision making is indisputable in this era. Surgical pathology laboratories are expected to deliver quality histopathology reports, timely. Quality is an intangible entity, defined as totality of features and characteristics of a product or service that has the ability to satisfy stated and implied needs. For a pathologist, quality means accuracy of diagnosis, for a histo-technician, quality may mean good quality of sections and for a clinician, quality means whether he received all the desired information or not. Quality in laboratory is thus, rightly defined as accurate, timely and complete reports [1]. Quality Assurance (QA) is the management system or strategy to ensure integrity of data, with an aim of value addition. The Quality Management System (QMS) decides certain parameters which are assessed periodically as measures of quality in a lab. These parameters which have to be defined, described, recorded, reviewed and audited regularly by the laboratory are called QI. These are used for systematically monitoring and evaluating patient care. The areas for improvement identified are addressed. With the use of QI, the laboratory endeavors to continually improve the effectiveness of the QMS, including pre analytic, analytic and post-analytic processes [2,3]. The present study was conducted with the objective to evaluate these QI and their role in minimising error rates and improving the quality in histopathology. There have been studies on pre-analytical errors like mislabeling/grossing room errors but, to the best of our knowledge, this is the first such study done various QI involving preanalytical, analytical and postanalytical aspects that may help in improving and maintaining quality in surgical pathology.
Materials and Methods
This cross-sectional study was conducted in the National Accreditation Board for Testing and Calibration Laboratories (NABL) accredited histopathology section of Department of Pathology at a Tertiary Care Hospital and Teaching Institute located in Dehradun, Uttarakhand, India for a period of two and a half years (January, 2016 to June, 2018). This study was conducted according to the Helsinki Declaration of 1975. As there was no use of patient data, consent was not required.
A set of nine QI were formulated for histopathology laboratory as per ISO 15189:2012 guidelines and quality manual by a focused group discussion amongst the authorised signatories and Quality Manager. These parameters were reviewed biannually by Quality Manager and section-incharge [Table/Fig-1] [3]. QI-1 included record of all specimens which were unacceptable and hence, rejected. QI-2 included record of any misidentification, wrong labeling of specimen or form, incomplete requisition forms, unlabelled specimens and tissue without appropriate fixatives. QI-3 is the record of any delay in calibration and annual maintenance of instruments along with failure in timely change of chemicals as per standard operating procedure. QI-4 is the record of any minor and major discrepancy in EQAP cycles. QI-5 is the record of number of amended reports issued and their reasons. TAT i.e., time between accessioning specimen and the report being finalised is recorded for every case. QI-6 is the record of cases with delayed TAT with a note of the reason. QI-7 is the record of all complaints and feedbacks from users i.e., patients as well as clinicians. QI-8 is the record of any breech in safety of the laboratory and personnel. All the non-conformities were recorded separately along with a mention of corrective and preventive actions taken. QI-9 comprised of regular audits undertaken to assess all record maintenance and documentation. Statistical analysis was done using ANOVA test followed by Post-hoc Tukey’s test Statistical Package of Social Sciences (SPSS) version 18.0, (SPSS, Chicago, IL).
Quality Indicators (QI) in histopathology laboratory.
| Quality indicator (QI)-1 | Number of unacceptable samples |
|---|
| QI-2 | Number of errors at registration and/or accession |
| QI-3 | Completeness of equipments |
| QI-4 | Performance in External quality assurance program† |
| QI-5 | Number of amended reports |
| QI-6 | Delay in TAT |
| QI-7 | Complaints and feedback from users |
| QI-8 | Laboratory safety and environment |
| QI-9 | Effectiveness of document control system |
Results
During the study period, a total of 11012 specimens including small and large biopsies were submitted in the histopathology section of the laboratory. Mean specimen number was 2242.4±430 for every six months. The data of various QI are evaluated shown in [Table/Fig-2].
Quality indicators (QI) during the study period.
| Quality indicator | Jan-Jun 2016 N=1670 | Jul-Dec 2016 N=2690 | Jan-Jun 2017 N=1776 | Jul-Dec 2017 N=2379 | Jan-Jun 2018 N=2497 | Total N=11012 n; (%) |
|---|
| Number of unacceptable samples | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of errors at registration and/or accession | 1M*, 1m† | 2m | 0 | 0 | 1 m | 5 (0.05%) |
| Number of amended reports | 0 | 1 (M*) | 0 | 2 (m†) | 0 | 3 (0.03%) |
| Complaints and feedback from users | 0 | 0 | 1 | 1 | 0 | 2 (0.02%) |
| Performance in EQAS‡ | Satisfactory | Satisfactory | Satisfactory | Satisfactory | Satisfactory | Satisfactory |
| Delay in TAT | 98 | 121 | 110 | 99 | 73 | 501 (4.5%) |
| Laboratory safety and environment | 0 | 0 | 0 | 0 | 0 | 0 |
| Completeness of equipments | Maintained | Maintained | Maintained | Maintained | Maintained | Maintained |
| Effectiveness of document control system | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate |
*M: Major error; †m: Minor error; ‡EQAS: External quality assurance system
Quality Indicators (QI) in Preanalytic Phase
The Number of unacceptable samples (QI-1): None of the histopathology specimens were rejected.
The Number of errors at registration/accession (QI-2): As illustrated in [Table/Fig-3], the error rate was minimal, 0.05% with respect to the histopathology specimen number and a mean of 1±0.89 in every six months. A major error was reported only once during the total study period.
Number of errors at registration/accession.
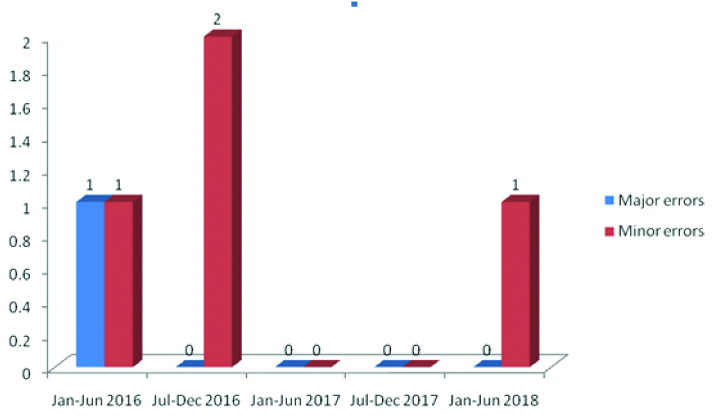
Completeness of equipments (QI-3): All the equipments were timely calibrated and proper annual maintenance was done. Thus, no non-conformity was recorded in this category.
Quality Indicators (QI) in Analytic Phase
Performance in EQAS (QI-4): The histopathology laboratory is enrolled in two EQAPs- Interlaboratory comparison program- histopathology (two monthly) and Interlaboratory quality assurance program (four monthly) which includes assessment of preanalytic and analytic aspects.
The results, as declared by the organising agencies, were satisfactory for both phases, in all the cycles of both the programs.
Quality Indicators (QI) in Post-analytic Phase
The number of amended reports (QI-5): As illustrated in [Table/Fig-4], the reporting of wrong results due to transcriptional mistakes was quite low, with an error rate of 0.03%. Corrected reports were issued in three cases during the study period.
Wrong reports and issuing amended reports.
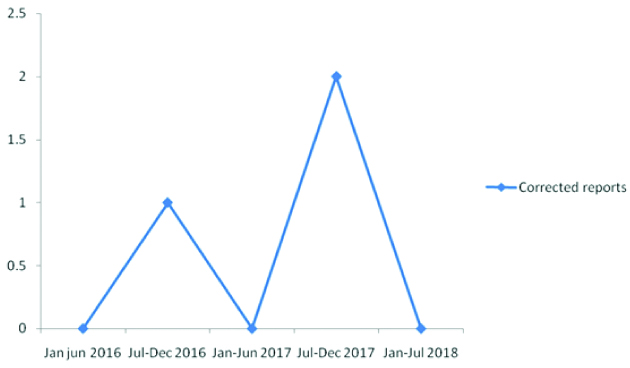
Delay in Turn Around Time (TAT) (QI-6)
Delayed TAT was noted in an average of 100±15.9 cases. It was observed that delay in TAT was the most critical and sensitive indicator (sensitivity= 98.04%) which was disturbed most frequently (4.5%) accounting for 98.04% of all errors, while others were only insignificantly disturbed. A detailed analysis of causes behind deranged TAT was done and is shown in [Table/Fig-5].
Factors contributing to delayed Turn Around Time (TAT).
| Phase | Causes | Jan-Jun 2016 N (%) | Jul-Dec 2016 N (%) | Jan-Jun 2017 N (%) | Jul-Dec 2017 N (%) | Jan-Jun 2018 N (%) | Total N (%) |
|---|
| Preanalytical | Incomplete clinical inputs | 8 (8.16) | 10 (8.26) | 9 (8.18) | 7 (7.07) | 8 (10.96) | 42 (8.38) |
| Preanalytical | Inadequate fixation | 9 (9.18) | 11 (9.09) | 14 (12.72) | 10 (10.10) | 5 (6.85) | 49 (9.78) |
| Preanalytical | Regross | 21 (21.43) | 25 (20.66) | 21 (19.09) | 19 (19.19) | 15 (20.55) | 101 (20.16) |
| Preanalytical | Processing problems | 13 (13.27) | 15 (12.40) | 17 (15.45) | 14 (14.14) | 10 (13.70) | 69 (13.77) |
| Preanalytical | Restain and Resection | 29 (29.59) | 39 (32.23) | 31 (28.18) | 28 (28.29) | 21 (28.76) | 148 (29.54) |
| Analytical | Special stains | 12 (12.24) | 14 (11.57) | 12 (10.91) | 13 (13.13) | 8 (10.96) | 59 (11.78) |
| Analytical | Immunohistochemistry | 3 (3.06) | 3 (24.79) | 4 (3.64) | 5 (5.05) | 4 (5.48) | 19 (3.79) |
| Postanalytical | Others | 3 (3.06) | 4 (3.30) | 2 (1.82) | 3 (3.03) | 2 (2.74) | 14 (2.80) |
| Total | 98 | 121 | 110 | 99 | 73 | 501 |
It was observed that preanalytical factors were the commonest cause of delay in TAT while the postanalytical factors were the least common. On applying ANOVA and Post-Hoc Tukey’s Test, it was inferred that preanalytical factors lead to statistically significant reason for delaying TAT (p=0.002) than analytical and postanalytical factors.
Complaints and Feedback from Users (QI 7)
During the study, only two complaints were received from the patients, which included delayed report generation and inconvenience during receiving of the specimen. Appropriate corrective action, root cause analysis and preventive actions were taken.
Laboratory Safety and Environment (QI-8)
Fire safety, equipment safety measures and measures to minimise and monitor formalin fumes exposure are practiced. No breech in lab safety was recorded during the study period.
Effectiveness of Document Control System (QI-9)
Regular audits undertaken revealed no deficit in document maintenance.
Discussion
Qualitattive Assesment (QI) is an objective measure that potentially evaluates all critical care domains i.e., patient care, safety, effectiveness, equity, timeliness and efficiency [4,5].
A laboratory should incorporate salient QI for monitoring its performance. This shall describe the evaluation of various aspects of a laboratory’s function such as but not limited to: sample collection and identification, transportation and processing, analysis and reporting of result, TAT, complaints, equipment downtime, uncertainty of measurements and performance in PT/EQA scheme [6].
Each laboratory should establish its own QIs. First, a reliable, measurable and reproducible QI has to be identified, which is then defined. Then, a protocol has to be established to evaluate it [6]. A proper record of these parameters should be reviewed periodically. Guidelines/standard operating procedures should be established for the same.
The list of QI should encompass and if possible, equally represent each phase all phases of analytical cycle i.e., pre analytical, analytical and postanalytical phases. ISO 15189:2012 does not specify the frequency of reviewing these parameters, but in authors’ experience, six monthly or atleast an annual review is sufficient [3,7]. The frequency of audit/review can be scaled as per the risk and occurrence management results [8]. A designated Pathologist/section-incharge or the Quality Manager of the laboratory should review QIs at decided intervals. Often, QI have to be individualised for each section of laboratory for example, the indicator like number of haemolysed samples or clotted samples, uncertainty of measurement etc., are valid for biochemistry and haematology sections while grossing errors and fixation errors are for histopathology section.
Similarly, stringent criteria can be followed in rejecting samples in haematology and biochemistry sections but the same cannot be applied for surgical pathology because reacquiring the surgical specimen is difficult and may affect patient care. Complying with this, there is a policy of not rejecting a surgical pathology specimen. In case of mislabeled or unlabelled specimens, the issue is resolved with the concerned personnel (clinican/resident/nursing staff), following which the specimens are accepted. Rao S et al., also followed similar policy of zero rejection rate in histopathology section [9]. Besides one of the accreditation bodies (NABL) also mentions in their guidelines “Histopathology specimens should not be rejected on grounds of poor specimen integrity. They should be accessioned & remarks be incorporated in the gross, microscopic descriptions and diagnostic interpretation as appropriate. In the case of specimen mislabeling or issues in specimen identification and traceability, the specimen shall not be accepted for testing without reconciling all issues. In the intervening period, the specimen shall not be discarded. Appropriate temporary labeling and if necessary, processing of the specimen may also be undertaken” [6].
Francis DL et al., classified specimen labeling errors as class 1 (only typographical), 2 (minor error) and 3 (significant error), depending on the resultant impact on patient care. Incorporation of a new specimen labeling system using Radiofrequency Identification Technology decreases the number of specimen labeling errors- 0.09% to 0.02% of class 3 errors [10]. Errors at registration or accession were less in number in the present study. This is possibly due to stringent use of two independent patient identifiers ie patient name and unique ID number generated at the reception.
The rate of issuing an amended report was low, varying from none to once in three months. Evaluating the clinical details properly before reporting and Intradepartmental Consultation (IDC) in difficult cases helps in maintaining the accuracy. Lind AC et al., also reported prospective peer review, i.e., review by a second pathologist before release of reports, to be quite beneficial in improving diagnostic accuracy in lieu of slightly extra time being spent [11]. Slight carelessness during typing or checking of typed reports may ruin all the meticulous work and lead to disastrous results. During the study period, there was a major error, which led to the issuing of a wrong result. Corrective action was taken and as a preventive action, a policy of two tier checking of typed reports- once by the resident doctor and second, by authorised signatory before releasing the report was incorporated. This helped to reduce number of wrongly finalised reports.
Weekly peer-review of randomly and retrospectively selected 2-3 cases, in terms of the overall quality of slides, diagnostic accuracy, the TAT and accuracy of Systematised Nomenclature of Medicine (SNOMED) coding, helped greatly in assessing and improving performance in surgical pathology [12].
In the present study, TAT was found to be the most commonly deranged QI and seems to be most sensitive of all indicators. TAT might be the only parameter which the laboratory consumers use to assess the efficiency of laboratory [4]. Further analysis of the causes of deranged TAT may help in identification of lacunae in the laboratory management system and hence, their rectification. It was also noted that preanalytical factors were statistically significant reasons behind delayed TAT. Root cause analysis of the problems and making certain amendments in standard operating procedures helped in improving TAT e.g., introducing the protocol of step cuts for small biopsies and suitable special stains as per the differential diagnosis mentioned on the requisition form at the time of grossing wherever possible. In view of greater number of grossing problems, seminars and teaching sessions were undertaken for the residents. This helped in reducing the grossing errors.
Layfield LJ and Anderson GM reported that most of the mislabeling errors occur in the grossing room [13]. On the other hand, Nakhleh RE et al., reported in a multi-institutional study that the mislabeling errors occurred mostly while tissue cutting (30.4%) followed by labeling the blocks (21.7%), pre-accessional stage (20.9%), accession (12.4%) and during grossing (10.2%) [14]. In current study, the rate of mislabeling errors was minimal.
Regular Continuing Medical education (CME), clinico-pathological conferences, emphasising clinicians and nursing staff on providing complete clinical details and adequate fixation helped in reducing the TAT and improving the accuracy of reports. Zuk JA et al., also observed that 1/5th of the requests had incomplete or absent clerical and clinical details which led to wastage of time [15].
Many a times neglected complaints and feedback from the users ie patients and clinicians, also helps to look upon the aspects which a pathologist may overlook. Working upon the issue raised helps in improving the quality of services provided by the laboratory.
It is important to record the non-conformities in laboratory daily so that timely corrective and preventive actions can be undertaken after thorough root cause analysis (i.e., Occurrence Management) [8]. A record of all this helps the lab and lab personnel to improve the quality of results generated.
The consistent evaluation of all the QI helped the authors to reduce the overall error rate including delay in TAT over the period of study in histopathology section [Table/Fig-2,5].
It is recommended that QI should also be formulated for Histopathology laboratory as is done for other sections of laboratory. These QIs should be recorded and reviewed periodically for improving and maintaining quality of reports generated.
Limitation(s)
This study was conducted at one centre only; hence, the results cannot be generalised.
Conclusion(s)
QI are important in minimising the error rate in laboratory. Although, there can be many possible QI in a laboratory, still the list has to be individualised according to the section concerned. Modifications can be done and new QI may be added to the list with time and increasing experience of the laboratory. Periodical review of QI help in understanding the flaws in the analytic cycle and appropriate corrective and preventive actions undertaken accordingly helps in improving the quality in histopathology laboratory.
*M: Major error; †m: Minor error; ‡EQAS: External quality assurance system