The RBD is a frequently, recognised type of parasomnia, in which person appears to be acting out their dreams [1]. It is a disabling condition, and leads to significant morbidity among its sufferer and is included in the DSM-5 as type of parasomnia [2]. Cause of RBD is thought to be idiopathic in majority, and secondary to obstructive sleep apnoea, narcolepsy, Parkinson’s disease, lewy body dementia, multiple system atrophy, and in the setting of certain medications such as antidepressants, among others [3]. Various studies, report around 46.5% to 80% of the cases on antidepressant treatment to be having RBD [4,5]. Among antidepressants, case reports and studies have found linkage of RBD with SSRI and SNRI [6-8]. TCAs have also been reported in case reports to cause RBD [6-8]. However, linkage of TCA has been less reported with RBD as compared to SSRIs and SNRIs which could be related to lesser usage of TCAs in clinical setting [4]. Also, sleep disorders are sometimes not reported by patients themselves, and neither adequately assessed due to time constraints by clinicians so, chances of missing less severe sleep disorders including RBD is high [8]. Also, with rise in the number of patients, needing antidepressants (including clomipramine) treatment, it is prudent, to study the sleep disorders, caused by them [9]. Since, RBD is a disabling condition, its early identification and appropriate management is necessary.
The present study was planned to understand the link between treatment with clomipramine and development of RBD. The study aimed at finding socio-demographic, and clinical correlates of RBD and to compare these parameters with those who didn’t develop RBD on treatment with clomipramine. No study has been reported to our knowledge, from this part of the world.
Materials and Methods
A case control study was conducted at outpatient unit of psychiatry department at a tertiary care multispecialty hospital, attached to a medical college in India. Data collection was done from February 2018 to January 2019. Patients coming to psychiatry OPD were informed about study through banner struck on the walls. Support staff of OPD also screened the patients on clomipramine during the registration process and encouraged such patients to participate in the study. Sample size was calculated based on the prevalence of previous study [10,11].
Inclusion criteria: All patients who presented to the psychiatry outpatient unit on the duty days of consultant author in the study, belonging to either gender and of any age, who were taking clomipramine, prescribed for any reason is included.
Only those participants who were willing to give written informed consent were included.
For Case Group: Patients with RBD were diagnosed by DSM-5 RBD.
For Control Group (NRBD group): Patients who were taking clomipramine but didn’t have diagnosis of RBD were included.
Since in the study, a case of RBD from among 5 to 10 participants on clomipramine (Mean 7.5) was found, so every 7th case from among the participants who were on clomipramine but didn’t have RBD, were included in the NRBD group.
Exclusion criteria: Presence of any psychotic disorder or any severe psychiatric disorder which could make the participation impossible in the study, substance use disorder (other than nicotine), severe cognitive impairment or any other serious or chronic medical illness like tuberculosis, diabetes, HIV, cancer etc., and those who were not willing to give written informed consent were excluded.
Tools of Assessment
Socio-demographic profile sheet
Details like age, sex, occupation, marital status and socio-economic status were taken on a socio-demographic profile sheet.
Clinical profile sheet
Details like current medications, dosage, duration of intake, presence of any sleep disorder and time since onset was noted in clinical profile sheet.
MINI version 5
Diagnosis of the psychiatric disorder was assessed using MINI version 5 using first the screening question and then detailed interview to evaluate for the presence and type of psychiatric disorder [12].
DSM-5
Diagnosis of RBD was based on DSM-5. The DSM-5 diagnostic criteria for RBD require presence of repeated arousals, vocalisations and motor behaviours, occurring well after the patient has slept. Also, the arousals shouldn’t be associated with any disorientation or confusion. It must lead to significant distress/impairment in functioning and shouldn’t be attributable to any substance use or medical condition. DSM-5 also mentions a requirement of polysomnography to confirm the association of behaviour disorders with Rapid Eyeball Movement (REM) sleep. However, in the diagnostic feature it is not an absolute requirement and a clinical diagnosis of RBD can still be made which can be later be confirmed by polysomnography. In present study, subjects who were of any age, receiving clomipramine for any diagnosis and who reported sleep disturbance were interviewed in detail to assess for the REM sleep behaviour disorder.
Study Procedure
Once any participant on clomipramine treatment was identified, they were sent to the senior resident for further assessment. Inclusion and exclusion criteria were applied. Participants were then assessed for presence of any sleep disorder by asking routine questions to assess them. Once, the participant was screened positively for possible presence of any sleep disorder, then detailed interview for RBD was done and the diagnosis of RBD was made on the basis of DSM-5 criteria for RBD. When the data for subjects in the RBD group was collected, selection of the cases in NRBD group was made, based on selection of every 7th participant among the cases who were on clomipramine but no diagnosis of RBD was made. Patients of both groups were interviewed further for socio-demographic and clinical profile on the semi-structured proforma. Further the patients were screened on MINI-5 for current ICD-10 psychiatric diagnosis.
Statistical Analysis
Data was analysed using Statistical Package for the Social Sciences (SPSS) Statistic 20.0 (IBM SPSS Statistics, New York, United States). Descriptive statistics was used for socio-demographic and clinical variable parameters. Normality of data was assessed using Pearson’s coefficient. Chi-square test and independent-t-test was used to make the group comparisons. Pearson correlation coefficient was used to make correlational analysis. Having RBD or not having it was taken as dependent variables and socio-demographic along with clinical variables were taken as independent variable. All tests were two-tailed. The value of p<0.05 was considered as statistically significant.
Results
Around 164 patients on clomipramine during the study period were analysed. Out of these 164 patients, sleep problems were found in 54 of the patients. These were evaluated in detail for the presence of RBD which was found in 20 participants, and were included in the RBD group. Every 7th case from 144 participants (34 having some sleep problems but not qualifying for diagnosis of RBD and 110 not having any sleep problems) who didn’t had RBD, were included in the NRBD group, to make a total of 20 participants [Table/Fig-1].
Flow chart of participant inclusion in the study.
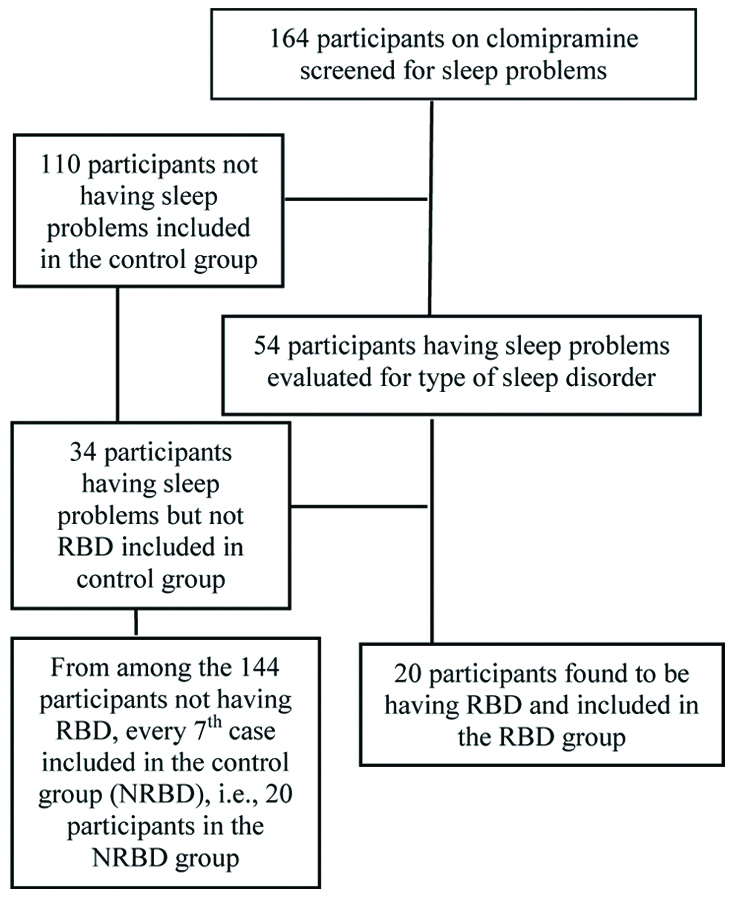
Mean age of the participants in RBD group was 36.650 (standard deviation±7.882) years and in the NRBD group was 34.250 (standard deviation±7.132) years. About 14 in the RBD group and 13 in the NRBD groups were female. No significant group differences were found on the socio-demographic parameters of the two groups on Chi-square and Independent t-test [Table/Fig-2].
Group comparisons of the socio-demographic characteristics.
| Variable | RBD n=20 (%) | NRBD n=20 (%) | Chi-square/Independent t-testTest statistics, df, p-value |
|---|
| Age (Mean±SD) | 36.650±7.882 | 34.250±7.132 | 0.403, df=38, p=0.405 |
| Sex | 0.114, df=1, p=0.736 |
| Male | 6 (30%) | 7 (35%) |
| Female | 14 (70%) | 13 (65%) |
| Marital status | 0.784, df=1; p=0.376 |
| Single | 2 (10%) | 4 (20%) |
| Married | 18 (90%) | 16 (80%) |
| Occupation | 0.143, df=1; p=0.705 |
| Employed | 15 (75%) | 16 (80%) |
| Nonemployed | 5 (25%) | 4 (20%) |
| Socio-economic status | 1.232; df=2; p=0.540 |
| Upper | 2 (10%) | 3 (15%) |
| Upper middle | 9 (45%) | 10 (50%) |
| Lower Middle | 6 (30%) | 6 (30%) |
| Upper Lower | 2 (10%) | 1 (5%) |
| Lower | 1 (5%) | 0 |
RBD-Rapid eyeball movement sleep behaviour disorder; NRBD-No rapid eyeball movement sleep behaviour disorder. Socio economic status classified according to Kuppuswamy’s socio-economic scale
About 18 in the RBD and 16 in the NRBD group had a primary diagnosis of Obsessive Compulsive Disorder (OCD). No significant group differences were seen with regard to primary diagnosis (p=0.572) and co-morbidity (p=0.170). Significant group differences were noted in duration of clomipramine treatment (p=0.026). Significant group differences were found with regard to dosage of Clomipramine in RBD and NRBD group (p=0.016) [Table/Fig-3].
Differences in the diagnosis and duration of RBD treatment.
| Variable | RBD (n=20) | NRBD (n=20) | Chi-square/Independent t-test Test statistics, df, p-value |
|---|
| Primary diagnosis | 1.118, df=2; p=0.572 |
| Obsessive compulsive disorder | 18 (90%) | 16 (80%) |
| Depressive episode | 1 (5%) | 3 (15%) |
| Anxiety disorder | 1 (5%) | 1 (5%) |
| Co-Morbidity≠ | 3.538, df=2; p=0.170 |
| None | 11 (55%) | 15 (75%) |
| Depressive episode | 9 (45%) | 4 (20%) |
| Anxiety disorder | 0 | 1 (5%) |
| Nicotine use | 3 (15%) | 4 (20%) |
| Mean duration of treatment with clomipramine (weeks) | 7.350±11.202 | 21.250±24.399 | 5.361, df=1; p=0.026 |
| Mean dosage of clomipramine in mg (Mean±SD) | 75±36.274 | 51.25±39.299 | 1.800, df=5, p=0.016 |
| Duration of RBD* (Mean±SD) | 16.4±11.118 Range (3-40) |
RBD-Rapid Eyeball Movement Sleep Behaviour Disorder, *Duration in days from starting or changing the dosages of clomipramine after which RBD developed; ≠Comorbidity was more than one in few patients; p<0.05 was considered as statistically significant
Odds ratio risk assessment of socio-demographic factors for RBD development showed higher risk with female sex (1.077; 95% CI=0.700-1.657), married (1.125; 95% CI=0.865-1.464) and un-employed status (1.250; 95% CI=0.392-3.986) [Table/Fig-4].
Odds ratio of RBD development with socio-demographic factors.
| Variable | Odds ratio | 95% Confidence interval |
|---|
| Sex |
| Male | 0.857 | 0.349-2.102 |
| Female | 1.077 | 0.700-1.657 |
| Marital status |
| Single | 0.500 | 0.103-2.428 |
| Married | 1.125 | 0.865-1.464 |
| Occupation |
| Employed | 0.938 | 0.671-1.310 |
| Un-employed | 1.250 | 0.392-3.986 |
| Socio-economic status* |
| Upper | 0.861 | 0.385-1.928 |
| Middle | 1.148 | 0.577-2.285 |
*For computation SES as classified on Kuppuswamy’s scale of upper, and upper middle taken as upper and lower middle, upper lower and lower taken as middle
Pearson correlational analysis showed that mean duration of RBD development correlated negatively with age (r=-0.479, p=0.034) and dosage of clomipramine (r=-0.095, p=0.690).
Discussion
The present study was an attempt to find the factors, related to the development of RBD on clomipramine. This study, to our knowledge, is first from the area where this study was conducted, to report findings, on this topic. The study found a higher prevalence rate of RBD, 12.195%, than reported in a previous study of 6% [10]. One study, on large number of subjects reported a lower prevalence of 3.98% patients of RBD with antidepressants [11]. DSM-5 mentions prevalence rate of RBD as 0.38-0.50% in general population and higher in persons on antidepressant treatment [2]. Present study finding, of higher prevalence rate of RBD, could be explained by the fact, that it included those patients who were already on some, or other antidepressant medication in which dose change or addition of clomipramine was done, so it could be possible that RBD, developed in patients already at risk of developing it. Clomipramine being TCAs, also have anticholinergic properties of varying degree and contribute to the reduction of REM sleep, mainly by reducing the effects of acetylcholine in the basal forebrain during REM sleep. Acetylcholine has potentiating effect on REM via activation of “REM-on” cells in the pontine tegmentum. This action, could possibly explain the REM sleep behaviour disorder greater than with SSRIs and SNRIs [7]. Many of the patients, assessed for the symptoms, could have been distressed by other side-effects of the drug, so they might have positively responded for the sleep disorder question put before them for initial assessment. Higher prevalence rates with clomipramine usage shows, clear association of antidepressant uses and development of RBD. As it was earlier thought, to be related, mainly to neurodegenerative conditions, toxicity or metabolic disturbances, alcohol withdrawal, infections, or traumatic lesions affecting brainstem structure [12] but present study findings, clearly shows the link between clomipramine (antidepressant) usage and RBD, as has been shown in few case reports and studies [7,13-15]. Also, the mean age of RBD presentation in present study was 36.65. RBD was previously thought to be an illness of elderly. Present study finding of lower age of onset is consistent with a recent review reporting change in the demographic of RBD. RBD no longer remains a disease predominantly of elderly but can affect young too with antidepressant uses [16].
The present study, did not find any significant differences, in the psychiatric co-morbidity in patients on clomipramine developing RBD than those who did not. As, in study sample, patients prescribed clomipramine were predominantly of OCD with depression as co-morbidity. So, depression as co-morbidity does not seem to increase the chances of developing RBD on clomipramine in patients of OCD. However, a prospective study with larger sample size can further clarify on this finding. In a study, on Parkinson’s disease found higher rates of RBD in patients suffering from co-morbid mood and anxiety disorders [17]. Another study, also reported greater chance of developing RBD on multiple medications and more psychiatric morbidities [18]. Also, majority of the patients developing RBD, were on higher dosage of clomipramine of 50 mg or more so judicious use of the drug in cases of OCD needs to be highlighted. A similar finding was reported in a review, where they found higher dosage to be associated with greater chance of developing parasomnias [19]. In present study, mean duration after which the RBD developed since the introduction or dose change of clomipramine was 16.4 days. Also, the mean duration with clomipramine treatment was significantly lower in patients who developed RBD than those who didn’t. RBD develops early in the cases with introduction or dose changes of clomipramine. Few studies, have also demonstrated similar finding of development of RBD after new antidepressant introduction or dose changes [18,19]. This study was an attempt to systemically examine the association of RBD development with clomipramine usage and would add to the sparse literature available on the subject. The study used detailed interviewing, and use of MINI to ascertain the diagnosis. Also, the study excluded the patients with medical illness which may be independent risk factor for RBD.
Limitation(s)
Firstly, as it was a case control study and participants were examined once so the persistence of symptoms and true relation could not be assessed with clomipramine dose changes and effects of other antidepressants, though a comparative study probably allowed for an even distribution of these factors between the two groups. Secondly, the sample size was too small for the results to be generalisable to other patients, a sample of convenience was chosen rather than the sample size as the study initially but since RBD is a rare condition and during the study period only 20 participants could be found having RBD on clomipramine so results were published for the same. Thirdly, polysomnography could not be used to ascertain a diagnosis of RBD due to non-availability of the instrument in our department, so it could be possible that few of the cases could actually be having some other parasomnias which could be found in polysomnography study. The prevalence rates for sample size estimation were calculated based on studies in western countries. Lastly, the prevalence rates of RBD were not available from Indian studies.
Conclusion(s)
The study was carried out to find some of the clinical correlates of RBD. Diagnosis for which clomipramine is started does not seem to have any impact on development of RBD. Co-morbid depression or anxiety disorder with OCD seems to have no impact on developing RBD on clomipramine. Higher dose of clomipramine is associated with development of RBD. RBD develops within a week or upto 6-7 weeks of starting or changes in the dosage of clomipramine. It should alert the clinicians for this disabling side-effect, of clomipramine and thus should be used wisely and development of RBD should be monitored in all the cases on clomipramine.
RBD-Rapid eyeball movement sleep behaviour disorder; NRBD-No rapid eyeball movement sleep behaviour disorder. Socio economic status classified according to Kuppuswamy’s socio-economic scale
RBD-Rapid Eyeball Movement Sleep Behaviour Disorder, *Duration in days from starting or changing the dosages of clomipramine after which RBD developed; ≠Comorbidity was more than one in few patients; p<0.05 was considered as statistically significant
*For computation SES as classified on Kuppuswamy’s scale of upper, and upper middle taken as upper and lower middle, upper lower and lower taken as middle