Impact of Efflux Pump Inhibitor Carbonyl-Cyanide M-Chlorophenylhydrazone in Multidrug Resistant Acinetobacter Species Isolates from Sterile Body Fluids
Shahid Raza1, Hitender Gautam2, Bhavna Maheshwari3, Sarita Mohapatra4, Seema Sood5, Benu Dhawan6, Arti Kapil7, Bimal Kumar Das8
1 Post Doctoral Fellow, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
2 Associate Professor, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
3 PhD Student, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
4 Associate Professor, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
5 Professor, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
6 Professor, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
7 Professor, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
8 Professor, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Hitender Gautam, Associate Professor, Department of Microbiology, Teaching Block, 2nd Floor, Room No. 2075A, New Delhi, India.
E-mail: drhitender@gmail.com
Introduction
Antimicrobial resistance of Acinetobacter baumannii (A. baumannii) are rapidly emerging, becoming non-responsive to most of the commonly prescribed antibiotics and leaving us with few treatment options and galloping treatment costs.
Aim
To study the effect of Efflux Pump Inhibitor (EPI) Carbonyl Cyanide 3-Chlorophenylhydrazone (CCCP) on Multidrug Resistance (MDR) A. baumannii isolates from different sterile body fluids.
Materials and Methods
A total of 40 Acinetobacter species isolated from different sterile body fluids i.e., Cerebrospinal Fluid (CSF), ascitic fluid, pleural fluid, and peritoneal fluid were collected and identified by Matrix Assisted Laser Desorption/Ionisation-Time Of Flight (MALDI-TOF), Biomerieux, France. Minimum Inhibitory Concentration (MIC) of A. baumannii was determined by automated VITEK-2 Antimicrobial Susceptibility Testing (AST) system (Biomerieux, France). In addition, MIC of the isolates, grown on Mueller-Hinton Agar (MHA) plate with 15 μg/mL with EPI CCCP (Sigma Aldrich, US) was determined. For Tigecycline, MIC was determined by Broth Microdilution (BMD) method.
Results
Out of 40 isolates, 34 (85%) were A. baumannii and 6 (15%) were Acinetobacter junii. Most of the Acinetobacter spps were MDR and only susceptible to few antibiotics. Most effective antibiotic was Tigecycline 25 (73.52%) followed by Co-trimoxazole 10 (29.41%). Similarly, Out of 40 isolates, 2 to 64 folds reductions in MIC was observed due to CCCP in 10 (25%) isolates for various antibiotics. Likewise, for Tigecycline, 2 to 4 folds reductions in MIC value (One strain changed from intermediate to sensitive) was observed by VITEK-2 AST which corroborated with reduction in MIC by BMD after addition of CCCP.
Conclusion
MDR A. baumannii are spreading rapidly. There is the need to overcome the antimicrobial resistance by investigating resistance inhibiting substance that will help to restore antimicrobial susceptibility and bringing back the existing antibiotics in prescription.
Acinetobacter baumannii, Broth micro-dilution, Efflux pump inhibitors, Minimum inhibitory concentration, Multidrug resistance
Introduction
A. baumannii is a major cause of various human infections such as bacteremia, meningitis, pneumonia, urinary tract infection, skin and soft tissue infection [1] throughout the world, and it is gaining due public health concern because of emerging MDR strains during the last few decades [2]. We are heading towards post-antibiotics era where alarming number of previously curable infections are changing into non-curable and are threatening to life [3]. However, antimicrobial resistance is the natural phenomenon, irrational use of antibiotics boosts-up the emergence of drug-resistant strains [4]. A. baumannii was susceptible to most of the antibiotics till 1970s. Drug resistance in A. baumannii can be intrinsic or acquired mostly through the acquisition of plasmids, transposomes or integrons which harbors clusters of genes encoding resistance to myriad families of antibiotics [5-8]. Transition of extra chromosomal material renders over expression of efflux pump that causes MDR [9] due to reduction in drug accumulation inside bacteria, and resulting in the increased MIC. Currently, efflux pumps are the newest and one of the most complicated bacterial resistance mechanisms that played a crucial role on drug resistance in A. baumannii [10]. Till date, it has been proven that five different families of efflux pump are present in A. baumannii i.e., ATP Binding Cassette (ABC) transporters, Resistance Nodulation-Cell Division (RND), Multidrug Toxic Composite Extrusion (MATE) transporters, Small Multidrug Resistance (SMR) and Major Facilitation Super family (MFS). Most common efflux pump families in A. baumannii responsible for MDR are ABC and RND [11,12].
Some molecules i.e., synthetic or natural have the potential to act specifically on efflux pump to restore the action of antimicrobial agents, known as EPIs [13] i.e., CCCP. Bacterial cell envelope has a crucial role in arbitrating resistance to antibiotics through its physiological properties, efflux pump and porine channels. More attention has been drawn to protonophores i.e., CCCP that reduces ATP production and increase membrane permeability in bacteria [14-16] by interfering with the transmembrane electrochemical gradient and proton motive force. In addition CCCP offers good reversal effect of drug resistance on A. baumannii [17]. This study was designed and conducted to observe the effect of CCCP on MIC of various antibiotics used for the treatment of A. baumannii from different sterile body fluids.
Materials and Methods
A cross-sectional study was designed and conducted at the Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India from June 2018 to September 2019. Total 40 representative MDR isolates of Acinetobacter species from various sterile body fluids i.e., CSF, pleural fluid, ascitic fluid and peritoneal fluid were collected and identified by MALDI-TOF. Inclusion criteria were collection of isolates only from sterile body fluids. Isolates were further processed for the determination of MIC for various antibiotics and was determined by automated VITEK-2 AST system as per manufacturer instruction (Biomerieux, France).
Bacterial Identification by MALDI-TOF
Sterile body fluids (ascitic, CSF, peritoneal fluid and pleural fluid) were inoculated on Blood, chocolate and Mac conkey agar plate and were incubated at 37°C for 24-48 hours. Smear of isolates grown on culture plate were made on MALDI-TOF slide. A 0.5 μL matrix (α-cyano-4 hydroxycinnamic acid) was added on the smear and kept it for one minute at room temperature. Slide was kept in MALDI-TOF (VITEK-MS, Biomerieux, France) machine for acquisition and identification [18].
Determination of Minimum Inhibitory Concentration (MIC)
MIC of A. baumannii was determined by automated VITEK-2 AST system (Biomerieux, France). An isolated colony of A. baumannii was picked up from the culture plate and mixed in normal saline. Turbidity was measured in Turbidometer (Biomerieux, France) and was adjusted to 0.5-0.6 CFU/mL as per manufacturer instruction. 145 μL bacterial suspension was transferred to the tube containing 3 mL normal saline and was mixed properly. VITEK Card N281 was used for AST and MIC determination. Result of the MIC was taken after 18-24 hours of incubation in VITEK-2 AST system. For the determination of MIC of Tigecycline (Sigma Aldrich, USA), along with VITEK-2 AST, BMD was also performed and taken as standard.
Addition of CCCP in MHA plate
To check and confirm the mechanism of efflux pump, CCCP was added to MHA plate. Final concentration of CCCP (Sigma Aldrich, USA) in MHA was maintained 15 μg/mL [19]. All the isolates were subcultured on MHA plate with CCCP. Then MIC was measured again by VITEK-2 AST system for all the A. baumannii isolates grown on MHA plate. In addition, for Tigecycline (Sigma Aldrich, USA), MIC was also determined by BMD (which is considered as reference standard for determination of MIC) in the presence of efflux pumps inhibitor, CCCP (CCCP, Sigma Aldrich, USA). Result of the MIC performed in VITEK-2 AST system in the presence of CCCP and without CCCP was compared. Similarly, MIC result of Tigecycline with CCCP by VITEK-2 AST system and BMD were also compared.
Statistical Analysis
Data were entered with coding and analysed in Microsoft Excel Version 10. Percentage of Acinetobacter species and their antibiotic susceptibility result was calculated.
Results
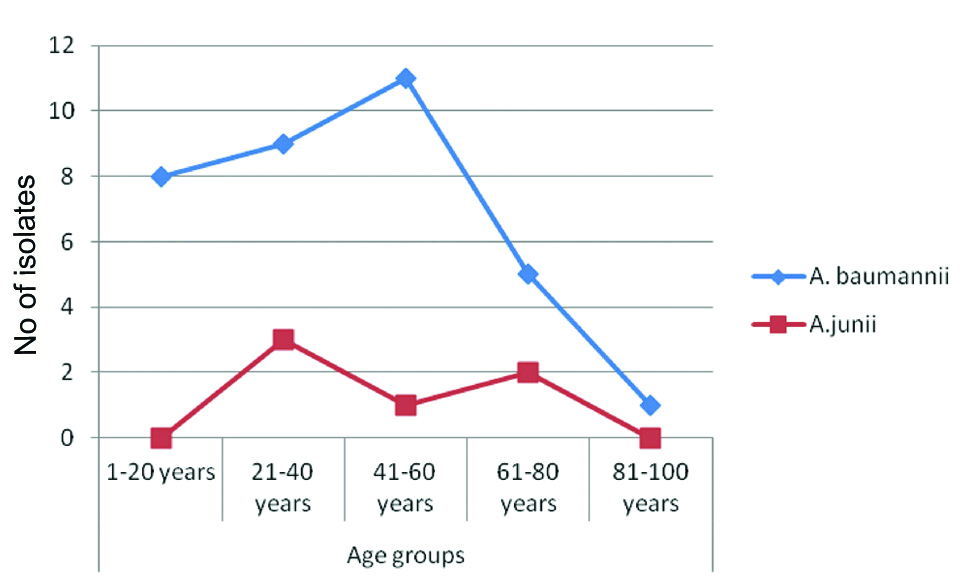
The experiment result was interpreted taking A. baumannii isolates from all age groups. A total of 40 isolates of Acinetobacter species were collected from different sterile body fluids. Of the total, 31 (77.5%) were male and 9 (22.5%) were female. The mean age of the patient was 40.27±20.50 years (Min 3-Max 93 years). Most of the A.baumannii (12, 35.29%) were present in 41-60 years of age group as shown in [Table/Fig-1]. Out of 40 isolates, 34 (85%) were A. baumannii and 6 (15%) were A. junii. Distribution of Acinetobacter species in different samples is shown in [Table/Fig-2].
Showing the distribution of Acinetobacter species in different age group.
Showing distribution of Acinetobacter species in different samples.
| Isolates | Samples | Total |
|---|
| CSF | Pleural fluid | Ascitic fluid | Peritoneal fluid |
|---|
| Acinetobacter baumannii | 12 (35.29%) | 11 (32.35%) | 07 (20.59%) | 04 (11.76%) | 34 |
| Acinetobacter junii | 02 (33.33%) | 03 (50.0%) | 01 (16.67%) | - | 06 |
| Total | 14 (35.0%) | 14 (35.0%) | 08 (20.0%) | 04 (10.0%) | 40 |
A. baumannii and A. junii showed different resistance pattern. Most of the A. baumannii were MDR and only susceptible to few antibiotics. Most effective antibiotic against A. baumannii was Tigecycline 25 (73.52%) followed by Co-trimoxazole 10 (29.41%) shown in [Table/Fig-3].
Showing the antibiotic resistance pattern of Acinetobacter baumannii and A. junii.
| Antibiotics | Isolates |
|---|
| Acinetobacter baumannii (34) | Acinetobacter junii (6) |
|---|
| S | I | R | S | I | R |
|---|
| Piperacillin/Tazobactam | 1 (2.94%) | 1 (2.94%) | 32 (94.11%) | 6 (100%) | - | - |
| Ceftriaxone | 1 (2.94%) | 2 (5.88%) | 31 (91.17%) | 6 (100%) | - | - |
| Cefaperazone/Sulbactam | 3 (8.82%) | 1 (2.94%) | 30 (88.23%) | 4 (66.66%) | - | 2 (33.33%) |
| Cefepime | 4 (11.76%) | - | 30 (88.23%) | 3 (50.0%) | - | 3 (50.0%) |
| Imipenem | 3 (8.82%) | 2 (5.88%) | 29 (85.29%) | 3 (50.0%) | - | 3 (50.0%) |
| Meropenem | 3 (8.82%) | 2 (5.88%) | 29 (85.29%) | 6 (100%) | - | - |
| Amikacin | 5 (14.70%) | - | 29 (85.29%) | 6 (100%) | - | - |
| Gentamicin | 5 (14.70%) | - | 29 (85.29%) | 6 (100%) | - | - |
| Ciprofloxacin | 4 (11.76%) | - | 30 (88.23%) | 6 (100%) | - | - |
| Tigecycline* | 25 (73.52%) | 9 (26.47%) | | 6 (100%) | - | - |
| Trimethoprim+Sulphamethoxazole | 10 (29.41%) | - | 24 (70.58%) | 4 (66.66%) | - | 2 (33.33%) |
S=Sensitive, I=Intermediate and R=Resistance; *FDA interpretive criteria for enterobacteriaceae was used
Effect of EPI (CCCP) on various antibiotics were observed, Acinetobacter species were cultured on MHA with 15 μg/mL CCCP and MIC was determined by VITEK-2 AST system. Out of 40 isolates, 2 to 64 folds reductions in MIC value were observed in 10 (25%) isolates for various antibiotics. Maxn reduction was observed in Meropenem (64 fold) followed by Trimethoprim-sulphomethoxazole (32 fold) [Table/Fig-4].
Showing the change in MIC (μg/ml) value of the isolates grown on MHA with CCCP.
| Isolates | Antibiotics |
|---|
| Tigecycline | Trimethoprim+Sulphamethoxazole | Gentamicin | Imipenem | Meropenem | Cefepime |
|---|
| MIC 1 | MIC2 | MIC1 | MIC2 | MIC1 | MIC2 | MIC1 | MIC2 | MIC1 | MIC2 | MIC1 | MIC2 |
|---|
| A.baumanniiStrain no. 50 | 4 (I) | 2 | 160® | 80® | - | - | - | - | - | - | - | - |
| A.baumanniiStrain no. 14 | - | - | - | - | 16® | 4 | - | - | - | - | - | - |
| A.baumanniiStrain no. 15 | - | - | 160® | 20 | 4 | 1 | 8® | 4 | 16® | 0.25 | - | - |
| A.baumanniiStrain no. 53 | 2 | 0.5 | 320® | 20 | 16® | 2 | 16® | 0.25 | - | - | 64® | 4 |
| A.baumanniiStrain no. 42 | - | - | 160® | 20 | - | - | - | - | - | - | 64® | 32® |
| A.baumanniiStrain no. 43 | - | - | 160® | 20 | - | - | 8® | 0.25 | 16® | 0.25 | - | - |
| A.baumanniiStrain no. 13 | 4 (I) | 2 | - | - | - | - | - | - | - | - | - | - |
| A.juniiStrain no. 51 | - | - | - | - | 2 | 1 | 8® | 4 | - | - | 4 | 1 |
| A.juniiStrain no. 37 | - | - | 160® | 80® | - | - | 8® | 0.5 | 16® | 0.25 | 32® | 1 |
| A.baumanniiStrain no. 39 | - | - | - | - | 8® | 4 | - | - | - | - | - | - |
MIC1: Isolates cultured on MHA, MIC2: Isolates cultured on MHA with CCCP, ®: Resistant
In addition, significant reduction (8-fold) in MIC value was also observed in 2 (5%) isolates against Ciprofloxacin. Of the 2 isolates, one was A. baumannii and one was A. junii. Likewise, for Tigecycline, 2 to 4 fold reductions in MIC value (One strain changed from intermediate to sensitive) was observed by VITEK-2 AST which corroborated with reduction in MIC by BMD after addition of CCCP.
Discussion
Acinetobacter species are most important bacterial agent causing different infections in human. Globally, more attention is given on A. baumannii due to the emergence of MDR leaving only limited option for the treatment. In the present study, most of the antibiotics tested were resistance, ranging from 70-100%. However, most effective antibiotic was Tigecycline which was 100% susceptible in A. junii. However, in A. baumannii, 25 (73.52%) isolates were sensitive and 9 isolates (26.47%) were intermediate in sensitivity. It was followed by co-trimoxazole in which 10 (29.41%) and 4 (66.66%) isolates were susceptible in A. baumannii and A. junii, respectively. Finding of this study is in concordance with the various studies that has shown increasing resistant in A. baumannii against different antibiotics worldwide [20]. Likewise, 88.23% isolates were resistant to ciprofloxacin which was less than the finding of Asadollahi P et al., who showed 100% resistance of A. baumannii to ciprofloxacin [21] though Shi WF et al., and Leseva M et al., has shown 52% and 62% are resistant to ciprofloxacin [22,23]. A. baumannii exhibits intrinsic MDR to a gamut of antibiotics due to an innate expression of efflux pumps, chromosomally encoded enzymes, and low membrane permeability. Plethora of chromosomally encoded efflux systems and Outer Membrane Porins (OMPs) has been identified that are responsible for MDR in A. baumannii [24]. Increased efflux as a result of over expression of efflux pumps is a common mechanism of MDR in A. baumannii, and resistance to a wide range of antibiotics.
In addition, MIC of different antibiotics was also measured after culture of A. baumannii on MHA plate with CCCP and without CCCP. Significant finding in the reduction of MIC was observed in 25% of the isolates against various antibiotics. Maximum reduction in MIC was observed in meropenem (64 folds) followed by trimethoprim (32 folds). As CCCP acts as a protonophore that binds reversibly to protons (H+) and transport them across the cell membrane that causes membrane depolarisation, eradication of electrochemical concentration gradient and less production of ATP [14,25]. It may be the reason of MIC reversal as well as conversion of resistance strain to sensitive strain after inhibiting efflux pump activity which indicates the role of efflux pump as the main mechanism behind emergence of resistance strain of A. baumannii. In this study, BMD for Tigecycline in addition to VITEK-2 AST was also performed for the determination of MIC. There were 4-fold reductions in MIC (determined by BMD) of Tigecycline after addition of CCCP. Similar reduction was observed in MIC of Tigecyclin by Osei Sekyere J et al., [26]. Concurrent result was observed for the MIC of Tigecyline determined by VITEK-2 AST after addition of CCCP. We infer that CCCP reverses drug resistance in A. baumannii. Though, CCCP is an experimental agent with no therapeutic value clinically, further studies are essential to decode the mechanism underlying the reversal effect of CCCP to bring it to the therapeutic use.
Limitation(s)
Possible pre-analytical and analytical variables that can interfere with the study has to be mentioned.
Conclusion(s)
MDR A. baumannii possess great challenge to the health system. As efflux pump is the main mechanism for MDR that could spread rapidly among the A. baumannii isolates, there is an urgency to search for an EPI to counteract the alarming situation of MDR.
S=Sensitive, I=Intermediate and R=Resistance; *FDA interpretive criteria for enterobacteriaceae was used
MIC1: Isolates cultured on MHA, MIC2: Isolates cultured on MHA with CCCP, ®: Resistant
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? No
Was informed consent obtained from the subjects involved in the study? No
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jul 26, 2020
Manual Googling: Sep 16, 2020
iThenticate Software: Oct 23, 2020 (15%)
[1]. Zhu L, Yan Z, Zhang Z, Zhou Q, Zhou J, Wakeland EK, Complete genome analysis of three Acinetobacter baumannii clinical isolates in China for insight into the diversification of drug resistance elementsPLoS One 2013 8(6):e6658410.1371/journal.pone.006658423826102 [Google Scholar] [CrossRef] [PubMed]
[2]. Peleg AY, Seifert H, Paterson DL, Acinetobacter baumannii: Emergence of a successful pathogenClin Microbiol Rev 2008 21:538-82.10.1128/CMR.00058-0718625687 [Google Scholar] [CrossRef] [PubMed]
[3]. Spengler G, Kincses A, Gajdacs M, Amaral L, New roads leading to old destinations: Efflux pumps as targets to reverse multidrug resistance in bacteriaMolecules 2017 22:46810.3390/molecules2203046828294992 [Google Scholar] [CrossRef] [PubMed]
[4]. World Health Organization. Antimicrobial Resistance. 2014. Available online at: http://www.searo.who.int/thailand/factsheets/fs0023/en/ (Accessed October 22, 2018) [Google Scholar]
[5]. Seifert H, Boullion B, Schulze A, Pulverer G, Plasmid DNA profiles of Acinetobacter baumannii: Clinical application in a complex endemic settingInfect Control Hosp Epidemiol 1994 15:520-28.10.2307/301484027983345 [Google Scholar] [CrossRef] [PubMed]
[6]. Devaud M, Kayser FH, Bachi B, Transposon-mediated multiple antibiotic resistance in Acinetobacter strainsAntimicrob Agents Chemother 1982 22:323-29.10.1128/AAC.22.2.3236100428 [Google Scholar] [CrossRef] [PubMed]
[7]. Segal H, Thomas R, Gay EB, Characterization of class 1 integron resistance gene cassettes and the identification of a novel IS-like element in Acinetobacter baumanniiPlasmid 2003 49:169-78.10.1016/S0147-619X(03)00011-8 [Google Scholar] [CrossRef]
[8]. Poirel L, Menuteau O, Agoli N, Cattoen C, Nordmann P, Outbreak of extended-spectrum beta-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospitalJ Clin Microbiol 2003 41:3542-47.10.1128/JCM.41.8.3542-3547.200312904353 [Google Scholar] [CrossRef] [PubMed]
[9]. Yoon EJ, Courvalin P, Grillot-Courvalin C, RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: Major role for AdeABC over expression and AdeRS mutationsAntimicrob Agents Chemother 2013 57(7):2989-95.10.1128/AAC.02556-1223587960 [Google Scholar] [CrossRef] [PubMed]
[10]. Hood MI, Jacobs AC, Sayood K, Dunman PM, Skaar EP, Acinetobacter baumannii increases tolerance to antibiotics in response to monovalent cationsAntimicrobial Agents and Chemotheraphy 2010 54:1029-41.10.1128/AAC.00963-0920028819 [Google Scholar] [CrossRef] [PubMed]
[11]. Temgoua FTD, Wu L, Mechanisms efflux pumps of Acinetobacter baumannii (MDR): Increasing resistance to antibioticsJournal of Biosciences and Medicines 2019 7:48-70.10.4236/jbm.2019.71006 [Google Scholar] [CrossRef]
[12]. Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J, Call of the wild: Antibiotic resistance genes in natural environmentsNature Reviews Microbiology 2010 8:25110.1038/nrmicro231220190823 [Google Scholar] [CrossRef] [PubMed]
[13]. Cheesman MJ, Ilanko A, Blonk B, Cock IE, Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution?Pharmacognosy Reviews 2017 11:5710.4103/phrev.phrev_21_1728989242 [Google Scholar] [CrossRef] [PubMed]
[14]. Spindler EC, Hale JDF, Giddings TH, Hancock REW, Gill RT, Deciphering the mode of action of the synthetic antimicrobial peptide Bac8cAntimicrob. Agents Chemother 2011 55:1706-16.0.1128/AAC.01053-1021282431 [Google Scholar] [CrossRef] [PubMed]
[15]. Ni W, Li Y, Guan J, Zhao J, Cui J, Wang R, Effects of efflux pump inhibitors on colistin resistance in multidrug-resistant gram-negative bacteriaAntimicrob Agents Chemother 2016 60:3215-18.10.1128/AAC.00248-1626953203 [Google Scholar] [CrossRef] [PubMed]
[16]. Park YK, Ko KS, Effect of carbonyl cyanide 3- chlorophenylhydrazone (CCCP) on killing Acinetobacter baumannii by colistinJ Microbiol 2015 53:53-59.10.1007/s12275-015-4498-525557480 [Google Scholar] [CrossRef] [PubMed]
[17]. Nikasa P, Abdi-Ali A, Rahmani-Badi A, Al-Hamad A, In vitro evaluation of proton motive force-dependent efflux pumps among multidrug resistant acinetobacter baumannii isolated from patients at Tehran HospitalsJundishapur J Microbiol 2013 6(7):e869110.5812/jjm.6792 [Google Scholar] [CrossRef]
[18]. Raza MS, Das BK, Goyal V, Lodha R, Chaudhry R, Sood S, Emerging multidrug resistance isolates of hospital-acquired bacterial meningitis in a tertiary care centre in North IndiaJournal of Medical Microbiology 2019 68:1585-90.10.1099/jmm.0.00107231647400 [Google Scholar] [CrossRef] [PubMed]
[19]. Lina Li, Linga BD, Xian-Zhi Li, Distribution of the multidrug efflux pump genes, adeABC, adeDE and adeIJK, and class 1 integron genes in multiple-antimicrobial- resistant clinical isolates of Acinetobacter baumannii-Acinetobacter calcoaceticus complexInternational Journal of Antimicrobial Agents 2009 33:27-32.10.1016/j.ijantimicag.2008.06.02718790612 [Google Scholar] [CrossRef] [PubMed]
[20]. Fallah F, Noori M, Hashemi A, Goudarzi H, Karimi A, Erfanimanesh S, Prevalence of bla NDM, bla PER, bla VEB, bla IMP, and bla VIM Genes among Acinetobacter baumannii isolated from two hospitals of Tehran, IranScientifica (Cairo) 2014 2014:24516210.1155/2014/24516225133013 [Google Scholar] [CrossRef] [PubMed]
[21]. Asadollahi P, Akbari M, Soroush S, Taherikalani M, Asadollahi K, Sayehmiri K, Antimicrobial resistance patterns and their encoding genes among Acinetobacter baumannii strains isolated from burned patientsBurns 2012 38(8):1198-203.10.1016/j.burns.2012.04.00822579564 [Google Scholar] [CrossRef] [PubMed]
[22]. Shi WF, Jiang JP, Xu N, Huang ZM, Wang YY, Inhibitory effects of reserpine and carbonyl cyanide m-chlorine-phenylhydrazone on fluoroquinolone resistance of Acinetobacter baumanniiChin Med J (Engl) 2005 118(4):340-43. [Google Scholar]
[23]. Leseva M, Arguirova M, Nashev D, Zamfirova E, Hadzhyiski O, Nosocomial infections in burn patients: Etiology, antimicrobial resistance, means to controlAnn Burns Fire Disasters 2013 26(1):05-11. [Google Scholar]
[24]. Coyne S, Courvalin P, Perichon B, Efflux-mediated antibiotic resistance in Acinetobacter sppAntimicrob Agents Chemother 2011 55:947-53.10.1128/AAC.01388-1021173183 [Google Scholar] [CrossRef] [PubMed]
[25]. Yu Z, Cai Y, Qin W, Lin J, Qiu J, Polymyxin E induces rapid Paenibacillus polymyxa death by damaging cell membrane while Ca2+ can protect cells from damagePLoS ONE 2015 10:e013519810.1371/journal.pone.013519826252512 [Google Scholar] [CrossRef] [PubMed]
[26]. Osei Sekyere J, Amoako DG, Carbonyl Cyanide m-Chlorophenylhydrazine (CCCP) reverses resistance to colistin, but not to carbapenems and tigecycline in multidrug- resistant enterobacteriaceaeFront Microbiol 2017 8:22810.3389/fmicb.2017.0022828261184 [Google Scholar] [CrossRef] [PubMed]