In children, most of the diagnostic and therapeutic procedures require sedation or analgesia or both to achieve the degree of cooperation or immobilisation. Sedation of children for imaging procedures like MRI is often challenging. Procedure like MRI which is conducted in a tunnel or duct in a noisy environment can frighten the child leading to movement artefact causing repetition of the sequences, prolonging the procedure and therefore mandates for deep or conscious sedation [1,2]. Hence, sedation is essential for children aged between 01 (one) to 12 years.
Sedation required in the MRI suite is different than what we practice in the operation theatre or in the Intensive Care Unit (ICU). Thus, a deep level of sedation is required during MRI, which is medically induced state of unconsciousness. Patients remain unresponsive to the external stimuli like verbal command; essentially remain motionless during the procedure with posing less risk to the potential complications of deep sedations [3].
Sedation of children for MRI is challenging due to limited access to the patient when imaging is on, the nature of the MRI hardware which precludes easy access to head and airway and the incompatibility of monitoring devices inside MRI suite leading to inadequate or failed sedation, difficulty in maintaining haemodynamic and respiratory stability along with the potential complications like hypoventilation, apnoea, aspiration, increased intra cranial pressure, hypotension [4]. The success of sedation for MRI is measured by its safety (lack of adverse events like bradycardia) and the effectiveness [3,5]. There is always an argument over the suitable drug and it’s dosage for MRI sedation in children. Keeping in mind the goals of sedation in the paediatric patient by American Academy of Paediatrics Committee on Drugs for diagnostic and therapeutic procedures, various drugs have been used till date [6].
Ketamine is an N-Methyl-D-Aspartate (NMDA) receptor antagonist used as an anaesthetic, sedative and analgesic. It is commonly ignored as a sedative for MRI as it has an analgesic component which is not necessary for MRI. Ketamine can be a suitable option for procedures like MRI with an onset time of one to three minutes and duration of 15-30 minutes. It has been recommended as a useful agent for sedation in patients with respiratory risk factors [8].
Both these drugs have been used in various studies in combination for day care procedures and have provided promising results [9-11]. In this study, these drugs were proposed to be compared amongst for monitored anaesthesia care in a paediatric population undergoing non invasive procedure.
The aim of the study was to compare the sedative effects, cardiac effects {Heart Rate (HR)} and respiratory effects {Respiratory Rate (RR) and saturation} of DEX in comparison to Ketamine in children undergoing MRI. Primary objective was to compare the onset time of sedation and the recovery time with either of the drugs. Also, the secondary objective was decided to compare the need to supplement sedation during the procedure and adverse events like apnoea and desaturation associated in both the groups.
Materials and Methods
It was a cross sectional study carried out in a large teaching hospital from August 2017 to July 2018 after obtaining clearance from the hospital ethics committee (IEC 72| 2017 dated 23 May 2017) and written, informed parents consent.
Children between the age groups six month to six years in American society anaesthesiologist category (ASA) I and II undergoing elective diagnostic MRI were included in the study.
Children having congenital heart disease, history of (H/O) upper respiratory tract infection, pneumonia or episode of acute severe asthma in the preceding four weeks, H/O recent use of digoxin, alpha 2-agonist or psychotropic medications were excluded from the study. Also, children with H/O allergies to the study drugs predicted, anatomical difficult airway and procedures taking time of less than 45 minutes were excluded from the study.
Total 74 patients were registered for the study. Two groups were decided as Group “K” for children receiving Ketamine and Group “D” for children receiving DEX. Group allocation was done randomly based on the odd and even number of the reporting date for pre anaesthetic check-up in the Institution. Allocation of patients to either group was done by a clinician not involved in the study and same was kept concealed until data collection and analysis were completed. All children were allowed to take clear liquids up to two hour before sedation but food (including breast milk) intake was with held as per standard guideline for Nil Per Os. (NPO)
Baseline values were recorded for all children upon arrival in the preparation room in the MRI suite. A 22G (gauge/size) or 24G venous cannula was inserted in the dorsum of the hand which was prepared one hour prior with the application of the Eutectic Mixture of Lignocaine and Prilocaine (EMLA) cream. If the procedure was delayed IV fluids was administered as per maintenance rate in the pre-anaesthesia care unit at the MRI suite.
A loading dose of DEX (1 mcg/kg was given over 10 min) or ketamine (1 mg/kg) with glycopyrrolate 10 mcg/kg) was given intravenously (IV) followed by continuous infusion of DEX (0.2-0.7 mcg/kg /h) in Group D or ketamine (10-15 mcg/kg/min) in Group K.
Response to sound, verbal commands or tactile stimulation were evaluated and sedation level of children was measured after every 10 minutes with the help of Ramsay sedation scale [12].
The Ramsay scale assigns a score of 1-6 based on the clinical assessment of the level of sedation (1=anxious, agitated, restless; 2=awake, but cooperative, tranquil, orientated; 3=responds to verbal commands only). Scores 4-6 apply to sleeping patients and are graded according to the response to loud noise or a glabellar tap (4=brisk response; 5=sluggish response; 6=no response).
The children were taken into the MRI suite when reflecting stable haemodynamic and respiratory parameters with a Ramsay sedation score of five. Time starting from drug infusion till achieving Ramsay score of five is defined as onset of sedation.
If a Ramsay score of six was not achieved after 15 minutes of study drug infusion to the maximum dose determined in the study protocol or inadequate sedation occurred during MRI examination, a single rescue dose of midazolam 0.1 mg/kg IV was administered (to a maximum of 3 mg by titration) to the patients in both the groups.
Inadequate sedation was defined as difficulty in achieving the desired level of sedation and not able to complete the procedure because of movement during MRI examination. HR, SpO2 and RR were monitored continuously and recorded at 5-minutes intervals during the study period by the observer inside the MRI suite. All patients were maintained on spontaneous respiration with a target SpO2>90%. Oxygenation was done via a transparent face mask fitted adequately. If there was a drop in SpO2 below 90% for 30 seconds, patient was taken out of the MRI tunnel and target SpO2 was achieved by various techniques of maintaining airway patency, titration of oxygen flow and with the help of airway adjuncts. Once settled down, the procedure was continued and the study drug infusion was titrated accordingly. Once the procedure was over, patients were shifted out to the recovery room following discontinuation of the study drug infusion.
The time period from the discontinuation of the study drug infusion to spontaneous eye opening and recorded modified Aldrate score of 10/10 of the patient in the recovery room followed by the time to discharge from the Post-Anesthesia Care Unit (PACU) were recorded.
Circulation was judged by HR instead of BP. The time intervals from PACU discharge were determined.
Statistical Analysis
For the purpose of sample size calculation, the statisitically significant difference in the time of onset of sedation between the two groups, a previous study was referred to [13]. To detect an observed difference of 20% in between the groups, with a power of study 80% and a type I error of 0.05, the minimum sample size required was 26 in each group. Total number of allocation of patients in various groups were kept more than 30, assuming a drop out of 10% patients.
Nominal data (number of subjects with apnoea, saturation and rescue medication etc.,) were presented as number (n) and percentage (%). Continuous variables (e.g., age, weight, HR, RR etc.,) were expressed as mean (Mean) and Standard Deviation (±SD). Chi-Square test was applied for comparison of nominal data. For continuous variable, unpaired t-test was applied to compare between groups. Paired t-test was applied to compare within group findings (Pre Vs Post). Additional parametric as well as nonparametric analysis of the data was performed as deemed essential. The p-value of <0.05 was considered as statistically significant. The analysis of the data was performed using Microsoft excel and Statistical Package for Social Sciences (SPSS) (software version 13.0).
Results
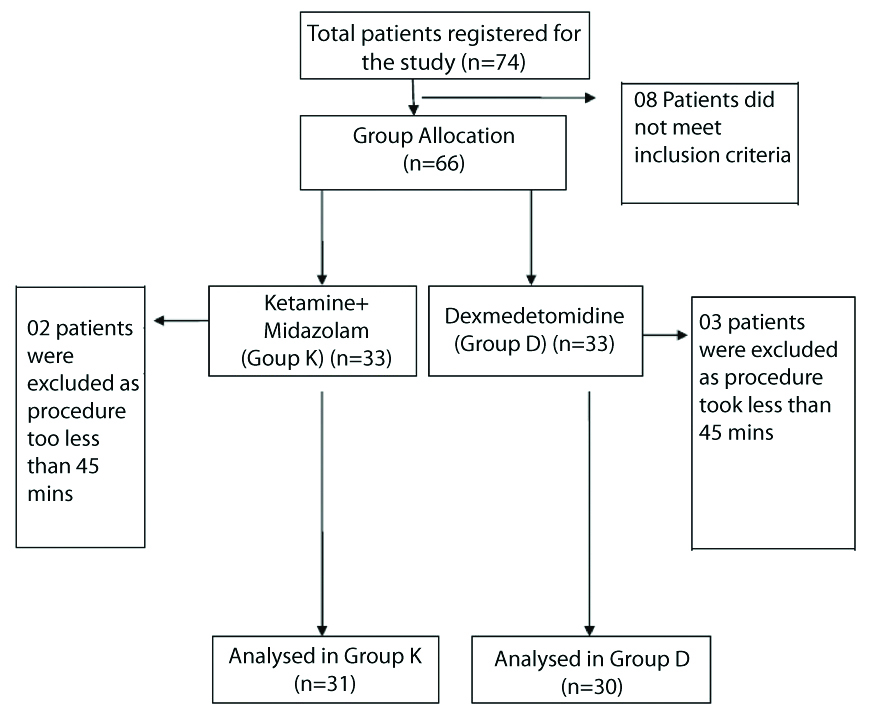
A total of 61 patients were analysed in the present study, 31 in Group K and 30 children in Group D [Table/Fig-1]. Both the groups were comparable in demographical distribution and the diagnosis for which an MRI was undertaken [Table/Fig-2]. Bilateral Sensorineural Hearing Loss (SNHL) and seizure disorder were the most common diagnoses in both the groups [Table/Fig-3]. HR and RR in both the groups prior to start of sedation (at 00 min) were comparable and not statistically significant. During sedation a decrease in both HR and RR from baseline was observed in both the groups. However, this decrease in both HR and RR when compared between the two groups were not statistically significant [Table/Fig-4].
Flow diagram of patient distribution.
Comparison of demographic variables.
| Parameter | Group K (n=31) | Group D (n=30) | Significance (p-value) |
|---|
| Mean | SD | Mean | SD |
|---|
| Age (years) | 4.64 | 3.16 | 4.88 | 2.83 | 0.76 |
| Weight (Kg) | 17.62 | 6.00 | 19.33 | 5.84 | 0.27 |
| Sex (M/F) | 18/13 | 21/9 | 0.5 |
Un-paired t test is applied. p-value is significant if <0.05
Comparison of diagnosis between Group K and Group D.
| Diagnosis | Group K (n=31) | Group D (n=30) | Grand total |
|---|
| Arnold chiarri malformation | 1 | 0 | 1 |
| B/L SNHL | 7 | 6 | 13 |
| Cerebellar ataxia | 0 | 1 | 1 |
| Cerebral palsy | 2 | 2 | 4 |
| Congenital hearing loss | 2 | 2 | 4 |
| Congenital short stature | 1 | 1 | 2 |
| Epilepsy | 0 | 1 | 1 |
| Facial nerve palsy | 1 | 1 | 2 |
| Floppy infant | 1 | 1 | 2 |
| Haemangioma-thorax abdomen | 0 | 1 | 1 |
| Hemiplagia | 0 | 1 | 1 |
| Hydrocephalus | 1 | 0 | 1 |
| Impaired hearing | 1 | 1 | 2 |
| Meduloblastoma | 1 | 2 | 3 |
| Meningomyocoele | 1 | 1 | 2 |
| Obstructive jaundice | 1 | 0 | 1 |
| Ophthalmic neuritis | 1 | 1 | 2 |
| Post meningitis sequlae | 0 | 1 | 1 |
| Precocious puberty | 1 | 0 | 1 |
| Seizure disorder | 5 | 5 | 10 |
| Short stature, failure to thrive | 2 | 1 | 3 |
| Spontaneous pneumthorax | 1 | 0 | 1 |
| Undescended testis | 1 | 1 | 2 |
| Grand total | 31 | 30 | 61 |
Un-paired t test is applied. p-value is significant if <0.05
Comparison of Heart Rate (HR) and Respiratory Rate (RR) between Group K and Group D.
| Parameter | Time points | Group K (n=31) | Group D (n=30) | Significance (p-value) |
|---|
| Mean | SD | Mean | SD |
|---|
| Heart rate (b/min) | minus 05 Min | 107.10 | 10.41 | 105.10 | 9.89 | 0.45 |
| 00 Min | 114.63 | 10.74 | 112.86 | 12.10 | 0.55 |
| 05 Min | 106.10 | 8.55 | 104.87 | 9.41 | 0.60 |
| 10 Min | 103.30 | 7.63 | 101.67 | 9.26 | 0.46 |
| 15 Min | 101.53 | 8.92 | 99.10 | 8.37 | 0.28 |
| 20 Min | 99.07 | 7.63 | 95.77 | 9.68 | 0.15 |
| 25 Min | 98.03 | 6.54 | 95.30 | 8.98 | 0.18 |
| 30 Min | 98.43 | 7.33 | 96.03 | 9.33 | 0.27 |
| 35 Min | 97.70 | 6.78 | 95.87 | 8.79 | 0.37 |
| 40 Min | 98.07 | 6.94 | 95.57 | 9.39 | 0.25 |
| 45 Min | 98.00 | 7.09 | 95.30 | 9.67 | 0.22 |
| 50 Min | 97.97 | 7.07 | 96.67 | 9.56 | 0.55 |
| 55 Min | 99.57 | 8.40 | 97.60 | 9.39 | 0.40 |
| 60 Min | 99.70 | 7.56 | 98.40 | 9.64 | 0.56 |
| Respiratory rate | 00-05 Min | 20.97 | 2.98 | 19.93 | 2.85 | 0.18 |
| 00 Min | 22.43 | 2.86 | 21.80 | 3.93 | 0.47 |
| 05 Min | 20.17 | 2.93 | 19.90 | 3.74 | 0.76 |
| 10 Min | 18.50 | 3.16 | 17.70 | 3.82 | 0.38 |
| 15 Min | 16.67 | 3.34 | 19.27 | 17.66 | 0.43 |
| 20 Min | 16.00 | 3.83 | 15.37 | 4.27 | 0.55 |
| 25 Min | 15.93 | 3.68 | 15.33 | 3.86 | 0.54 |
| 30 Min | 15.90 | 3.37 | 15.27 | 3.67 | 0.49 |
| 35 Min | 16.20 | 2.31 | 15.63 | 2.76 | 0.39 |
| 40 Min | 16.40 | 2.14 | 15.93 | 2.30 | 0.42 |
| 45 Min | 16.47 | 2.49 | 16.10 | 3.06 | 0.61 |
| 50 Min | 16.57 | 2.40 | 16.63 | 2.47 | 0.92 |
| 55 Min | 17.40 | 2.49 | 17.43 | 2.49 | 0.96 |
| 60 Min | 18.13 | 2.15 | 18.13 | 2.34 | 1.00 |
*Un-paired t-test is applied. p-value is significant if <0.05
Event of adverse reaction like desaturation and apnoea was not observed in any patient in either group. The saturation level from 0-60 minutes in both the groups, recorded at every 05 minutes interval, showed no statistically significant differences [Table/Fig-5]. Adequate sedation, as defined by obtaining a Ramsay Sedation Score of 6, was attained in all the patients in both the study groups. There were no cases of sedation failure or requirement for rescue sedation in any of the study subjects.
Comparison of saturation and events of apnoea between Group K and Group D.
| Parameter | Time points | Group K (n=31) | Group D (n=30) | Significance (p-value) |
|---|
| Mean | SD | Mean | SD |
|---|
| Saturation | 00-05 Min | 99.10 | 0.88 | 98.83 | 1.02 | 0.28 |
|---|
| 00 Min | 98.67 | 1.03 | 98.57 | 1.10 | 0.72 |
| 05 Min | 94.33 | 16.23 | 94.00 | 16.21 | 0.94 |
| 10 Min | 93.07 | 16.77 | 93.00 | 16.79 | 0.99 |
| 15 Min | 95.57 | 1.59 | 95.40 | 1.81 | 0.71 |
| 20 Min | 95.20 | 1.81 | 95.10 | 1.77 | 0.83 |
| 25 Min | 95.17 | 1.95 | 95.37 | 2.06 | 0.70 |
| 30 Min | 94.87 | 1.98 | 95.00 | 2.15 | 0.80 |
| 35 Min | 95.47 | 1.78 | 95.63 | 1.99 | 0.73 |
| 40 Min | 95.47 | 1.89 | 95.43 | 1.81 | 0.94 |
| 45 Min | 95.47 | 1.91 | 95.27 | 1.93 | 0.69 |
| 50 Min | 94.80 | 1.65 | 95.17 | 1.95 | 0.43 |
| 55 Min | 95.97 | 1.54 | 96.17 | 1.60 | 0.62 |
| 60 Min | 96.40 | 1.50 | 96.70 | 1.29 | 0.41 |
| Events of Apnoea | 00-60 Min (recorded at every 05 min interval) | Group K (n=31) | Group D (n=30) | Significance (p-value) |
| NIL | NIL | 1.00 |
*Un-paired t-test is applied. p-value is significant if <0.05
All the patients completed their MRI scan without any interruption. However, the onset of sedation (Mean±SD) in Group K was 6.30±1.32 minutes and 12.20±SD=2.01 minutes in Group D (p=0.001). The time to Modified Aldrete Score of 10/10 was higher in Group K. (Mean±SD; 21.10±1.84 minutes in Group K vs 13.73±1.89 minutes in Group D [Table/Fig-6]. This difference in between the groups was statistically significant (p=0.001).
Comparison of induction and recovery between Group K and Group D.
| Parameter | Group K (n=31) | Group D (n=30) | Significance (p-value) |
|---|
| Mean | ±SD | Mean | ±SD |
|---|
| Time To Ramsay Sedation Scale Score of Six (in Min) | 6.30 | 1.32 | 12.20 | 2.01 | 0.001 |
| Time To Aldrete Score 10/10 (in min) | 21.10 | 1.84 | 13.73 | 1.89 | 0.001 |
Un-paired t test is applied. p-value is significant if <0.05
Discussion
The study results indicated that the patients’ demographic were not statistically different between groups. Both groups had adequate procedural sedation (Ramsay sedation score of five) for MRI scan and 100% of the children in both the groups completed their scan without any interruption, interference or any complications. It had shown that both sedative drugs (DEX vs. ketamine) can be used safely in sedation for MRI. This finding is similar to the studies done using these drugs [14-16]. In a previous study, it was noted that the onset of sedation time was 19 minutes for DEX in MRI sedation [15]. In this study, the faster onset of sedation time by the study drugs (Mean±SD; 12.20±2.01 minutes) could be explained by the fact that here the accepted Ramsay score of five (5) was considered for the time to onset of sedation as opposed to the accepted Ramsay score of six (6) in the previous study [16].
In the present study, ketamine provided faster onset of sedation than DEX. The time to Ramsay Sedation Scale=5 was significantly higher in Group D (12.20±2.01 minutes) as compared to Group K (6.30 min±1.32 minutes) with a p-value=0.001 which is statistically significant. This finding is similar to previous study [14].
In the present study while analysing the recovery and achieving discharge criteria in both the groups it was observed that the time to Modified Aldrete Score 10/10 was significantly higher in Group K (21.10±1.84 minutes) as compare to Group D (13.73±1.89 minutes) (p-value=0.001). This finding is similar to the study by Eldeek AM et al., [14]. They reported that the recovery and the discharge time were longer in the DEX group [14]. Arian and colleagues reported a recovery time of 34 min with DEX in adults [17]. The recovery time was shorter in this study. This could be explained by the fact that the subjects were children and that the duration of infusion was shorter in present study.
Use of ketamine in paediatric age group was first published by Dachs and Innes in 1997 [18]. They have mentioned about similar result for the onset time for sedation with the use of ketamine for a similar dose. Ackwort JP et al., in their study have used 1 mg/kg intravenous ketamine and 0.1 mg/kg intravenous midazolam for procedural sedation in paediatric patients [19].
In this study, there was no requirement of supplemental sedation in both the groups. The procedure could be completed in both the groups without any events of inadequacy of sedation. In a previous study while comparing DEX and ketamine, they had to resort to rescue sedation in both the groups which is completely different observation in this study which could not be explained [14]. This finding can be verified if a future study with a larger population can be planned. Both the groups had comparable HR, RR and saturation throughout the study. Both groups however showed fall in HR, RR and SpO2 at and after 15 minutes as compared to the baseline values but the statistical significance cannot be commented as intra group analysis was not done. However, these parameters never went below the normal acceptable value for that age group and were not of any significant magnitude so as to warrant any interruption or intervention. Decreases in HR have been reported over time with DEX in children [14]. However, there were no instances of bradycardia requiring any intervention in this study. Some studies have also shown same type of findings with bradycardia after bolus doses of DEX [20,21]. But in this study, there was no bradycardia as the loading dose was administered over ten minutes and all the patients were pre medicated with Inj Glycopyrrolate 10 mcg/Kg IV. Ketamine has shown to decrease in HR in this study which may be reflex bradycardia. However, this fall in HR was never of clinical magnitude requiring intervention or interruption of the MRI scan.
Though respiratory events make up a large population for complications of sedation in children, some authors have reported that DEX did not affect RR and SpO2 [22]. However, some respiratory complications have been reported with large and rapid initial loading doses of DEX. Ketamine preserves ventilation, pharyngeal and laryngeal reflexes and does not create transient apnoea [23]. In this study, RR did decrease from the baseline in both the groups however this was not clinically significant and required no intervention during the two treatments.
Thus, together with the absence of any episodes of apnoea, bradycardia or desaturation suggests that neither DEX nor Ketamine depresses respiration excessively in children when used in the dose range and manner used in this study. None of the patients in this study had an episode of oxygen desaturation. This finding is consistent with other studies using DEX for sedation in paediatric patients [16,24,25].
Limitation(s)
MRI scans with a longer duration (approximately >45 minutes) were chosen. This exclusion of scanning procedures of smaller duration might have lead to a selection bias. In this study, the pre-procedural behaviour before administering drugs were not monitored or the blood pressure before, during or after the scan. One thought was that paediatric patients are more dependent on HR than on blood pressure to maintain cardiac output, hence monitoring of blood pressure would have been inconsequential. Some observations like no incidence of sedation failure in both the groups could not be explained. A larger study population can testify this finding.
Conclusion(s)
Ketamine shows early onset of adequate sedation but statistically significant delayed recovery and discharge as compared to DEX, thus making DEX a better alternative. However, both DEX and ketamine provide adequate sedation in procedures like MRI without any adverse events or requirement of rescue sedation.
Un-paired t test is applied. p-value is significant if <0.05
Un-paired t test is applied. p-value is significant if <0.05
*Un-paired t-test is applied. p-value is significant if <0.05
*Un-paired t-test is applied. p-value is significant if <0.05
Un-paired t test is applied. p-value is significant if <0.05