Metastatic Carcinoma to Breast from Renal Cell Carcinoma- A Rare Case Report with Unusual Cytology
Neelam Sood1, Shikha Chopra2
1 Consultant and Head, Department of Pathology, Deen Dayal Upadhyay Hospital, Delhi, India.
2 Senior Resident, Department of Pathology, Deen Dayal Upadhyay Hospital, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shikha Chopra, B132, 2nd Floor, DDA Flats, Mount Kailash Apartments, Opp. DPS School, East of Kailash, New Delhi-110065, Delhi, India.
E-mail: drshikhadawar@gmail.com
Metastatic carcinoma to breast is an uncommon entity compared to the primary breast malignancies. Renal Cell Carcinoma (RCC) metastasising to breast is very rare as it commonly metastasises to lung, liver or bone. An accurate diagnosis of Secondary Tumour is must, since the prognosis and treatment differs between primary and secondary tumours. Here, the authors present the case of a 55-year-old female patient who presented with lump in right breast measuring 5×4 cm. Mammographic findings showed relatively well-defined round irregular marginated heterogenous radioopaque mass in outer lower quadrant, Breast Imaging Reporting and Data System (BIRADS- IV B). Fine Needle Aspiration Cytology (FNAC) showed atypical cells arranged in cohesive clusters with focal papillary architecture, entangled in eosinophilic stroma and cellular stromal fragments. The cells were small with ill-defined cell borders, granular to focal vacuolated cytoplasm, mild anisocytosis and indistinct nucleoli. Stain for mucin was negative. The differential diagnosis offered were primary breast carcinoma with possibility of metaplastic carcinoma and metastatic carcinoma. A trucut biopsy showed fibrocellular stroma, islands of cells with clear cytoplasm separated by thin fibrous septa. Immunohistochemical (IHC) for pancytokeratin (panCK), CD10 and vimentin were positive and Estrogen Receptor (ER), Progesterone Receptors (PR), Human epidermal growth factor receptor 2 (HER2) were negative and the diagnosis of metastatic RCC was made. The cytology case reports in the literature have shown characteristic cytomorphological features composed of cells with abundant, finely vacuolated cytoplasm, moderate pleomorphism and prominent nucleoli. This case is unusual as it was predominated by small cells with granular cytoplasm. Therefore, it was concluded that trucut biopsy is mandatory for breast lump in patient with RCC since the cytological features on FNAC might not always be characteristic.
Granular cytoplasm, Microcalcifications, Mucicarmine stain, Trucut biopsy
Case Report
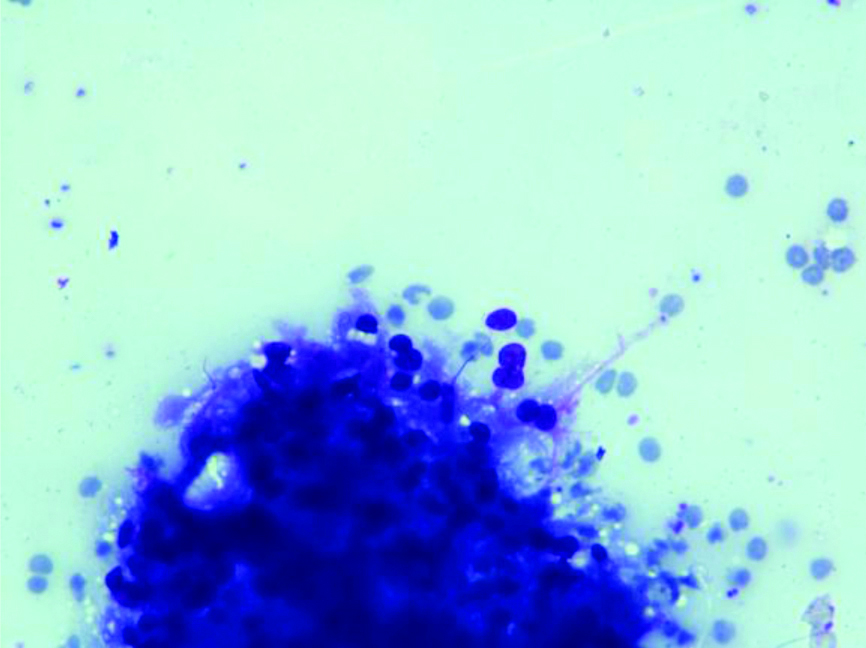
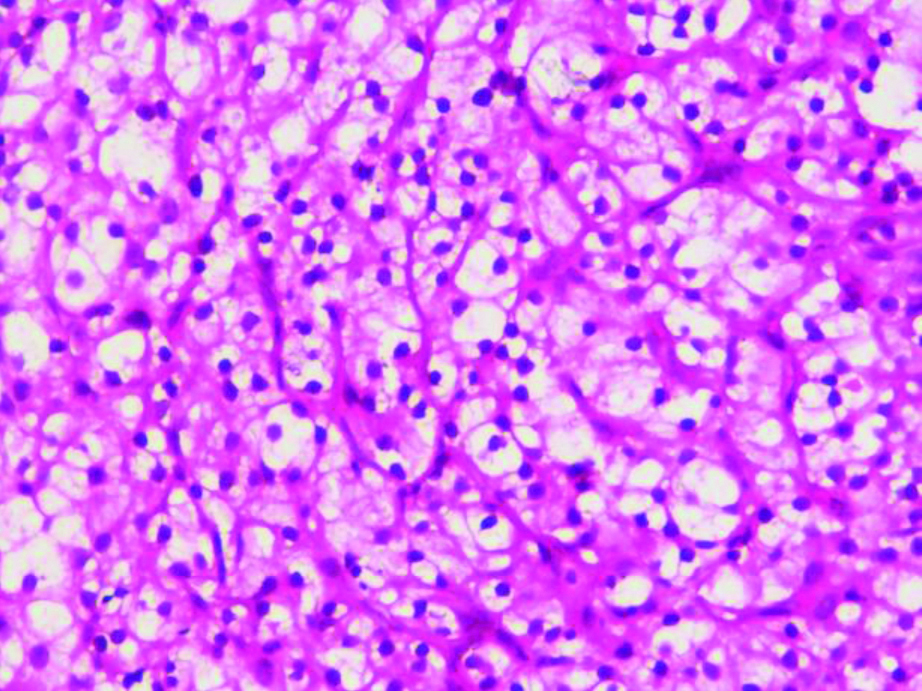
A 55-year-old female patient, presented with lump in the right breast measuring 5×4 cm of six months duration. There were no other complaints at the time of presentation and the past history was negative. The lump was well-defined, mobile and was noted in the lower outer quadrant. Mammographic findings showed relatively well-defined round irregular marginated heterogenous radioopaque mass in lower outer quadrant, categorised as BIRADS-IV B. Few tiny microcalcifications were also seen. FNAC was performed, smears from which showed atypical cells arranged in cohesive clusters with focal papillary architecture entangled in eosinophilic stroma and cellular stromal fragments. The cells were small with ill-defined cell borders, granular to focal vacuolated cytoplasm, mild anisocytosis and indistinct nucleoli [Table/Fig-1,2 and 3]. Mucicarmine stain was done on these smears which did not reveal any intracytoplasmic mucin. Cell blocks prepared from the fine needle aspirate, were non-contributory as the tissue was scanty and therefore, no special stain could be done on the cell blocks. The differential diagnosis offered on FNAC was primary breast carcinoma with possibility of metaplastic carcinoma and metastatic carcinoma. Further, tissue biopsy was advised for confirmation. The trucut biopsy showed fibrocellular stroma, islands of monomorphic cells separated by thin fibrous septa. The cells had clear cytoplasm, round nuclei with indistinct nucleoli [Table/Fig-4].
Cellular fragment comprising of small cells with ill-defined cell borders. (H&E x 400).
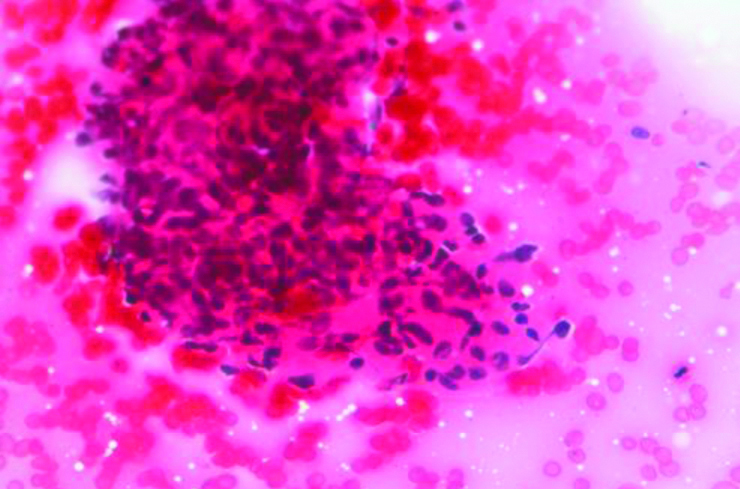
Cohesive cluster of atypical cells (Giemsa x 100).
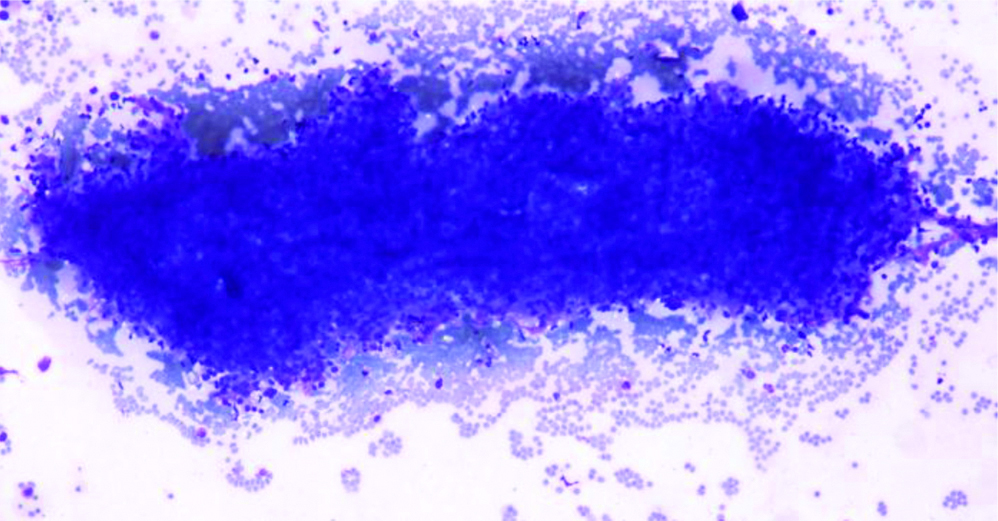
Cells showing granular and vacuolated cytoplasm (Giemsa x 400).
Islands of clear cells separated by fibrous septae. (H&E x 400).
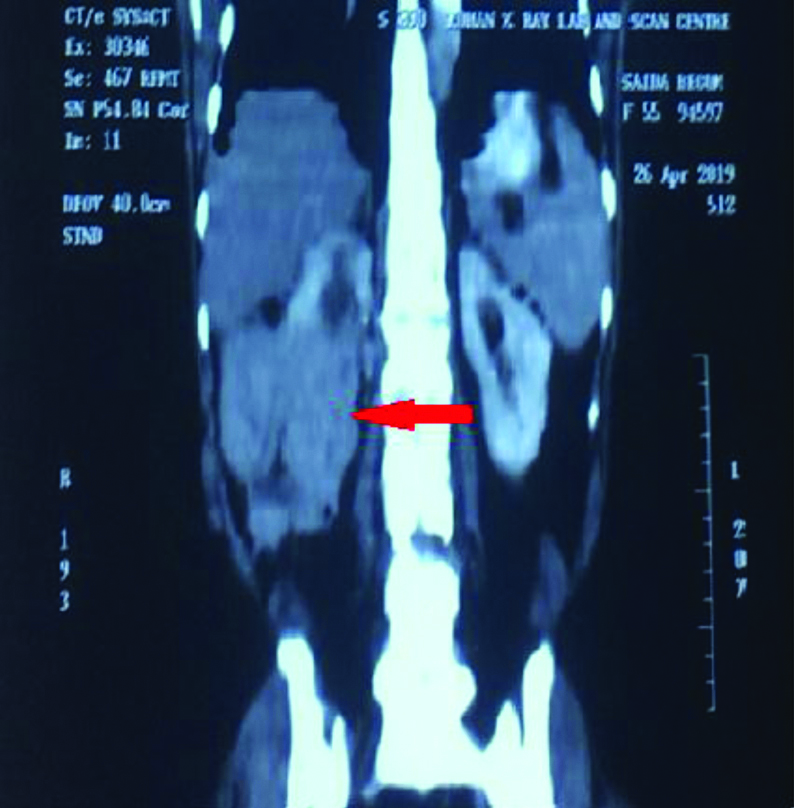
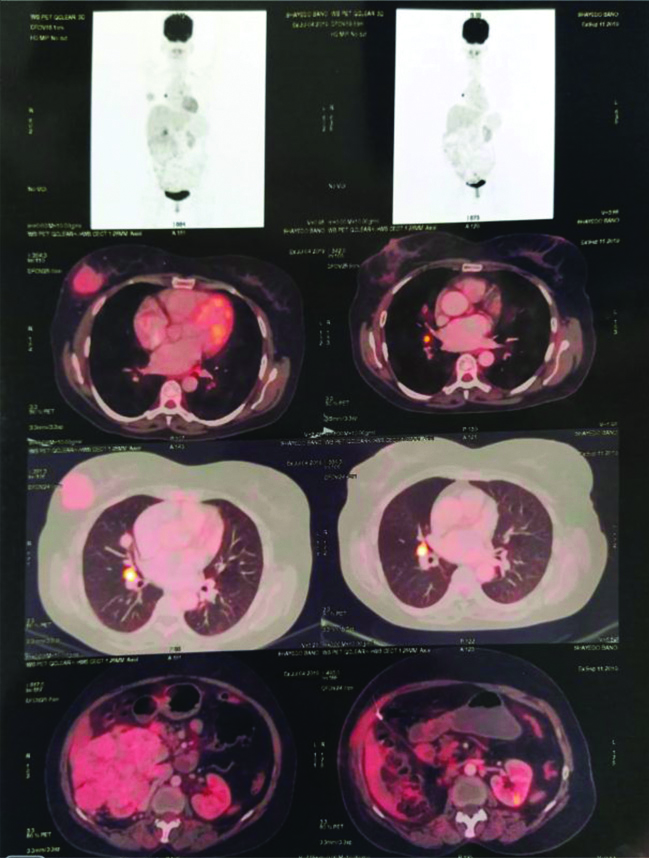
The histopathological differential diagnosis of primary low grade breast carcinoma and metastatic carcinoma were considered. Immunohistochemistry panel selected included ER, PR, Her2, pan CK, CD10 and vimentin. IHC for panCK, CD10 and vimentin were positive and ER, PR, her2 were negative. The IHC confirmed the diagnosis of metastatic carcinoma of renal origin. On re-examination, a mass was identified in the right renal fossa. High-Dose Contrast-Enhanced Computed Tomography (CECT) abdomen findings revealed a large lobulated, exophytic heterogeneously isodense, soft tissue mass lesion arising and involving the mid and lower pole of kidney, showing central necrosis with minimal peripheral calcific foci. The lesion measured 115×121×167 mm in size. Multiple vascular channels were noted at the upper and lower pole of the lesion. This was reported as right RCC [Table/Fig-5]. It was followed by whole body PET-CT scan which revealed metabolically active lesions in right breast, right kidney and right lung [Table/Fig-6]. The patient was then, referred to the higher center.
CECT whole abdomen shows right renal exophytic lobulated, enhancing necrotic mass lesion involving mid and lower pole (red arrow), measuring 115×121×167 mm in size and bulging outside the perinephric and gerotas fascia, most likely neoplastic lesion- RCC.
CECT: Contrast-enhanced computed tomography; RCC: Renal cell carcinoma
PET-CT study reveals metabolically active soft tissue density mass lesion arising from mid and lower pole of right kidney; right breast lesion and right lung nodule- Possiblity of metastatic renal cell carcinoma.
PET-CT: Positron emission tomography-computed tomography
Discussion
Metastatic carcinoma to breast is an uncommon entity compared to the primary breast tumours. This rare occurrence of breast metastases has been explained by the presence of large fibrous tissue having relatively poor vascular supply [1]. The most common extramammary sources of metastases are lymphoma, leukaemia and melanoma. Other uncommon sources include carcinoma of ovary, stomach, lung and rarely carcinoma of liver, endometrium, tonsil, pancreas, perineum, pleura, bladder and cervix [1,2].
RCC metastasising to breast is very rare as it commonly metastasises to lung, bone, lymph node, liver or adrenals [3]. The clinical manifestations of RCC are quite variable. About 30% of patients already have distant metastasis at the time of diagnosis [4]. A study done in metastatic RCC patients showed that only two out of 558 patients had RCC metastasis to the breast [5]. Few case reports, including one from India have been published in the literature showing breast metastasis from RCC [6-10].
Metastatic diseases of the breast can present as single or multiple lesions. These metastatic masses are often located in the subcutaneous plane or adjacent to the breast parenchyma and have relatively rich vascular supply. Radiologically, these lesions are often round to oval, well-defined, hypoechoic, non-spiculated, calcified or architecturally distorted. Most benign tumours also present with similar radiological features, therefore, these are not specific for metastatic tumours [11].
FNAC has shown to play a crucial role in the diagnosis of breast lesions. According to the literature, metastatic RCC to breast have shown characteristic cytomorphological features which are composed of cells having abundant, finely vacuolated cytoplasm with moderate pleomorphism and prominent nucleoli. Tumour cells may be arranged singly or in loose clusters, flat sheets, papillary fronds, alveolar pattern. Occasionally, the morphological pattern may be mixed. Three types of cells which can occur either exclusively or admixed together are clear cell, granular cell and oncocytic cell. The clear cells have been described as having abundant, fragile and finely vacuolated cytoplasm showing punched-out or bubbly vacuoles. The cytoplasm is so characteristic that it has been termed as ‘opaque with or without vacuolization and granulation’. This is to distinguish it from the so-called clear cells seen in histopathology, which gets denuded of their cytoplasmic contents during the histological process. Nucleus is partially extruded. They mostly have ill-defined cell borders which may sometimes be well-defined. The other type of cell, namely granular cells have moderately dense and granular eosinophilic or cyanophilic cytoplasm. The third type i.e., oncocytic cells have highly dense, compact and eosinophilic cytoplasm. The cell borders are well-defined. Rarely, RCC may show a diffuse spindled pattern [12].
This case is unusual as it was predominated by clusters of small cells with granular cytoplasm lacking the prominent punched out nucleoli; interspersed with few vacuolated cells. This was a diagnostic dilemma. Here, the trucut biopsy along with diagnostic CD10 positivity was helpful in reaching the final diagnosis. Therefore, trucut biopsy is mandatory in such cases.
Conclusion(s)
Cytomorphological features on FNAC in case of RCC might not always be characteristic. Therefore, cytopathologists should be familiar with the various features associated with subtypes of RCC. Further, histological corelation and ancillary studies including immunohistochemical markers should be done.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: May 07, 2020
Manual Googling: Aug 12, 2020
iThenticate Software: Oct 16, 2020 (12%)
[1]. Mun SH, Ko EY, Han BK, Shin JH, Kim SJ, Cho EY, Breast metastases from extramammary malignancies: Typical and atypical ultrasound featuresKorean J Radiol 2014 15(1):20-28.10.3348/kjr.2014.15.1.2024497788 [Google Scholar] [CrossRef] [PubMed]
[2]. Benveniste AP, Marom EM, Benveniste MF, Mawlawi OR, Miranda RN, Yang W, Metastases to the breast from extramammary malignancies- PET/CT findingsEur J Radiol 2014 83(7):1106-12.10.1016/j.ejrad.2014.04.01524844731 [Google Scholar] [CrossRef] [PubMed]
[3]. Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, Distribution of metastatic sites in renal cell carcinoma: A population-based analysisAnn of Oncol 2012 23(4):973-80.10.1093/annonc/mdr36221890909 [Google Scholar] [CrossRef] [PubMed]
[4]. Bennington JL, Beckwiht JB, Tumours of the kidney, Renal pelvis and Ureter. In Atlas of Tumour Pathology, 2nd SeriesWashington DC Armed Forces Institutes of Pathology 1975 :93-199. [Google Scholar]
[5]. Gravis G, Chanez B, Derosa L, Beuselinck B, Barthelemy P, Laguerre B, Effect of glandular metastases on overall survival of patients with metastatic clear cell renal cell carcinoma in the antiangiogenic therapy eraUrol Oncol 2016 34(4):167 [Google Scholar]
[6]. Botticelli A, De Francesco GP, Di Stefano D, Breast metastasis from clear cell renal cell carcinomaJ Ultrasound 2013 16(3):127-30.10.1007/s40477-013-0026-924432163 [Google Scholar] [CrossRef] [PubMed]
[7]. Falco G, Buggi F, Sanna PA, Dubini A, Folli S, Breast metastases from a renal cell carcinoma. A case report and review of the literatureInt J Surg Case Rep 2014 5(4):193-95.10.1016/j.ijscr.2014.01.01924632302 [Google Scholar] [CrossRef] [PubMed]
[8]. Gupta JD, Gupta D, Sen I, Chowdhury SP, Das A, Metastatic renal cell carcinoma presenting as breast mass in a maleJournal of Case Reports 2018 8(1):18-22.10.17659/01.2018.0006 [Google Scholar] [CrossRef]
[9]. Jin K, Wang J, Ye C, Xiong H, Xie B, Wang W, Breast metastasis from clear cell renal carcinoma: A case report and literature reviewInt J Clin Exp Med 2018 11(9):10116-20. [Google Scholar]
[10]. Ikarashi D, Ishida K, Kashiwaba M, Kato Y, Shiomi E, Takayama M, Sporadic breast metastasis derived from renal cell carcinoma: A case reportUrol Case Rep 2017 16:126-28.10.1016/j.eucr.2017.11.03229255682 [Google Scholar] [CrossRef] [PubMed]
[11]. Xu Y, Hou R, Lu Q, Deng Y, Hu B, Renal clear cell carcinoma metastasis to the breast ten years after nephrectomy: A case report and literature reviewDiagnostic Pathology 2017 12:7610.1186/s13000-017-0666-829096639 [Google Scholar] [CrossRef] [PubMed]
[12]. Mustaffa W, Husain S, Fine needle aspiration cytology of metastatic renal cell carcinoma- A case reportMed & Health 2006 1(1):75-80. [Google Scholar]