Introduction
Alcohol consumption is a major cause of liver disease worldwide. The amount of alcohol ingested is the most important risk factor for the development of Alcoholic Liver Disease (ALD). Osteopontin (OPN) is an extracellular matrix protein that is markedly up-regulated in patients with ALD.
Aim
To determine the best predictor of ALD among Serum Aspartate Transaminase (AST)/Alanine Transaminase (ALT) ratio, Alkaline Phosphatase (ALP), Gamma Glutamyl Transferase (GGT) and serum OPN.
Materials and Methods
Clinically diagnosed cases of ALD (N=60) with alcohol consumption ≥100 gm/day, for more than eight years and age and gender matched healthy controls (N=60) were recruited for the study. Estimation of AST, ALT, ALP and GGT were assayed by standard photometric methods in auto analyser ERBA-XL (EM-200) and plasma OPN was estimated by using commercial kit based on Enzyme-Linked Immunosorbent Assay (ELISA). Receiver Operator Characteristic (ROC) curve analysis was done to establish the best predictor of ALD among the markers.
Results
The parameters of the liver function tests such as AST, ALT, ALP were significantly increased in the cases (p<0.001) as compared to controls. In the study, there was a significant increase in the level of OPN and GGT in the patients with ALD (p<0.001) as compared to controls. OPN showed significant positive correlations with AST (r=0.76, p<0.001), ALT (r=0.64, p<0.001), ALP (r=0.68, p<0.001). Upon ROC analysis, OPN had the maximum area (0.998) under curve as compared to GGT and AST/ALT ratio.
Conclusion
OPN is a better predictor of ALD as compared to GGT and AST/ALT ratio.
Introduction
Alcohol consumption is the most common cause of cirrhotic chronic liver disease in India (34.3%) [1]. Heavy drinkers and alcoholics may progress from fatty liver to alcoholic hepatitis to cirrhosis and it is estimated that 10% to 15% of the alcoholics develop cirrhosis [2]. In addition to this, the burden of alcohol-related disease in the developed countries accounts for as much as 9.2% [3] of all disability-adjusted life years with alcohol-related cirrhosis being the second most common indication for liver transplantation in countries like Europe (40%) and Unites States of America (USA) (20%) [4]. The amount of alcohol ingested is the most important risk factor for the development of ALD. Intake of 80 grams of alcohol per day has been defined as “hazardous drinking” and consumption of amounts in excess of 80 grams significantly increases the risk of developing cirrhosis [5]. In hepatocytes, ethanol is metabolised into acetaldehyde by alcohol dehydrogenase, cytochrome P450 and catalase. This generates reactive oxygen species and causes lipid peroxidation, mitochondrial glutathione depletion and S-adenosylmethionine depletion. These lead to hepatocytic injury [6,7]. Acetaldehyde is highly toxic to hepatocytes because it promotes glutathione depletion, lipid peroxidation and mitochondrial damage [8,9]. Moreover, chronic alcohol ingestion leads to resistance to the lipogenic effects of insulin in addition to decreased ability of adipose tissue to take up and esterify Non-Esterified Fatty Acid (NEFA), thus increasing the circulating NEFA. Increased NEFA promotes hepatic steatosis through direct esterification in the hepatocytes and by inducing hepatic de novo lipogenesis through synthesis of Sterol Regulatory Element Binding Protein (SREBP) [10]. Furthermore, it induces hepatic insulin resistance and leads to release of pro-inflammatory cytokines which trigger Hepatic Stellate Cells (HSC) to synthesise collagen and cause fibrosis. Chronic alcohol consumption also increases the serum leptin levels causing HSC induced fibrosis and hepatic inflammation. Thus, prolonged alcohol intake can lead to ALD by affecting metabolic, endocrine and immune function leading to hepatic steatosis, inflammation and fibrosis.
The enzyme GGT is a membrane bound glycoprotein which catalyses the transfer of theγ-glutamyl group from γ-glutamyl peptides to other peptides, amino acids and water [11]. It occurs as a membrane bound enzyme in the kidney, pancreas, liver, spleen and small intestine. GGT is a microsomal enzyme which is present in hepatocytes, biliary epithelial cells, renal tubules, pancreas and intestine [12]. It is also present in cell membrane and is involved in glutathione metabolism. Raised serum activity of the enzyme has been reported in alcoholism, various forms of liver diseases including primary and secondary hepatic tumors, diabetes mellitus, cardiovascular disease, renal neoplasms, and the nephrotic syndrome [13]. OPN was first described as a phosphoprotein, secreted by a transformed cell line in 1979. The OPN gene is located on chromosome 4 region 22 (4q22.1) in humans. The protein is composed of about 300 amino acids and has two isoforms, a secreted form (sOPN) and an intracellular form (iOPN). It has various post-translational modifications such as phosphorylation, sulfation, glycosylation and proteolytic cleavage. It has an Arginine-Glycine-Asparate (RGD) domain, which binds with high affinity to integrins [14].
In a study conducted by Morales-Ibanez O et al., they suggested that hepatic expression and serum levels of OPN were markedly increased in alcoholic hepatitis compared to healthy controls and patients with other types of chronic liver diseases and correlated with short-term survival and disease severity [15]. Patouraux S et al., also postulated that serum OPN is progressively increased in liver fibrosis and associated with the stage of fibrosis in alcoholic patients and thus, it can be biomarker for significant fibrosis [16]. Hence, aim of the study was to determine the role of serum OPN as a biomarker in the diagnosis of ALD and compare it with AST/ALT ratio, ALP, GGT.
Materials and Methods
This was a hospital based cross-sectional study which was carried out in the Department of Biochemistry and Medicine, Faculty of Medicine and Health Sciences, SGT Hospital and Research Institute, SGT University, Gurugram, Haryana, India from November 2018 to May 2019. The ethical clearance for the study was taken from Institutional Ethics Committee (IEC letter no. IEC/FMHS/AC/29/11/2018-M.Sc dated-29.11.2018). Clinically diagnosed patients of ALD (N=60) in the age group of 20-60 years attending Medicine Outpatient Department (OPD) of SGT Hospital were included as the cases. Cases were defined based on medical history, physical examination and laboratory investigations. Age and gender matched healthy controls (N=60) were recruited from general population as they reported to the medicine OPD for routine health checkup. After explaining, the purpose and details of the study to all the subjects of both the groups, a written and informed consent was taken.
Inclusion Criteria
Alcohol intake ≥100 gm/day,
Duration of alcohol intake >8 years [17,18],
AST level 2-6 times raised as compared to ALT.
Exclusion Criteria
Viral hepatitis B and C,
Autoimmune hepatitis,
Drug induced hepatitis,
Copper and iron storage disease (Wilson disease, haemochromatosis),
Chronic smoking,
Patient on steroids, antihypertensive drugs, hypoglycaemic drugs or hormone replacement therapy,
Concomitant inflammatory disorders,
Renal disorders (ruled out on the bases of Kidney Function Test).
Laboratory Analysis
A total of 5 mL of venous blood was collected after 12-14 hours of fasting taking all aseptic precautions, out of which 3 mL was collected in plain vial and serum was separated by centrifuging at 3000 rpm for 10-15 minutes and 2 mL blood was collected in an EDTA containing vial for the estimation of OPN. Liver Function Tests of the subjects were estimated immediately and one aliquot was preserved at -20°C for the estimation of OPN within 1 month of collection of sample. Internal quality control for all the tests was performed using control materials obtained from ERBA, Germany. Estimation of AST, ALT, ALP and GGT was assayed by standard photometric methods in auto analyser ERBA-XL (EM-200) using commercially available kits. OPN was estimated by using commercial kit based on ELISA.
Statistical Analysis
The data recorded was entered in a spreadsheet and then statistical analysis were performed by using Statistical Package for the Social Sciences (SPSS) Version 21.0. Continuous variables were summarised in the form of means and standard deviations. Graphical data was presented by bar diagrams. Student’s independent t-test was employed for comparing continuous variables. Pearson’s correlations coefficient was applied to study the association of OPN with liver enzymes of ALD patients. ROC curve analysis was done to establish the best predictor of ALD among the markers. The p-value <0.05 was considered statistically significant for all the parameters.
Results
The mean age of the ALD patients was (53.06±4.38) years and for the healthy controls was (50.76±5.89) years [Table/Fig-1].
Demographic parameters of the subjects.
| Parameters | Cases (N=60) | Controls (N=60) | p-value |
|---|
| Sex | Male | 56 | 55 | 0.729 |
| Female | 04 | 05 |
| Age (years) | 53.06±4.38 | 50.76±5.89 | 0.016 |
Liver Function Tests
The parameters of the liver function tests such as AST, ALT, ALP were significantly increased in the patients with ALD (p<0.001) when compared to the healthy control subjects [Table/Fig-2].
Comparison of liver function tests among ALD patients and the controls.
| Parameters | Cases (Mean±SD) | Controls (Mean±SD) | t-value | p-value |
|---|
| AST (U/L) | 67.48±9.43 | 18.86±3.79 | 37.04 | <0.001 |
| ALT (U/L) | 36.58±5.81 | 20.98±5.02 | 15.72 | <0.001 |
| ALP (U/L) | 97.90±29.45 | 40.26±8.01 | 14.62 | <0.001 |
| AST/ALT ratio | 1.86±0.27 | 0.93±0.24 | 19.73 | <0.001 |
Student’s independent t-test applied; <0.05 statistically significant
AST: Serum aspartate transaminases; ALT: Alanine transaminases; ALP: Alkaline phosphatase; SD: Standard deviation
Markers of Alcoholic Liver Disease (ALD)
In the present study, significantly increased levels of OPN and GGT were found in the patients with ALD (p<0.001) when compared with the control subjects [Table/Fig-3].
OPN levels among ALD patients and control subjects.
| Parameters | Cases (Mean±SD) | Controls (Mean±SD) | t-value | p-value |
|---|
| GGT (U/L) | 46.14±9.06 | 28.96±9.28 | 10.25 | <0.001 |
| OPN (ng/mL) | 97.43±31.59 | 34.33±11.28 | 14.56 | <0.001 |
Student’s independent t-test applied; <0.05 statistically significant
GGT: Gamma glutamyl transferase; OPN: Osteopontin
Correlations of OPN with Liver Enzymes
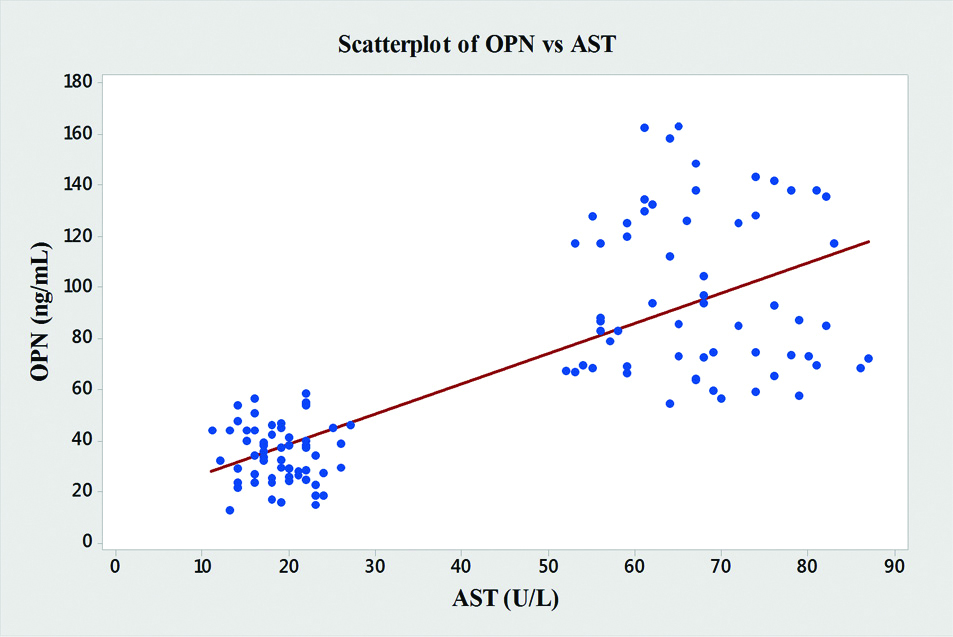
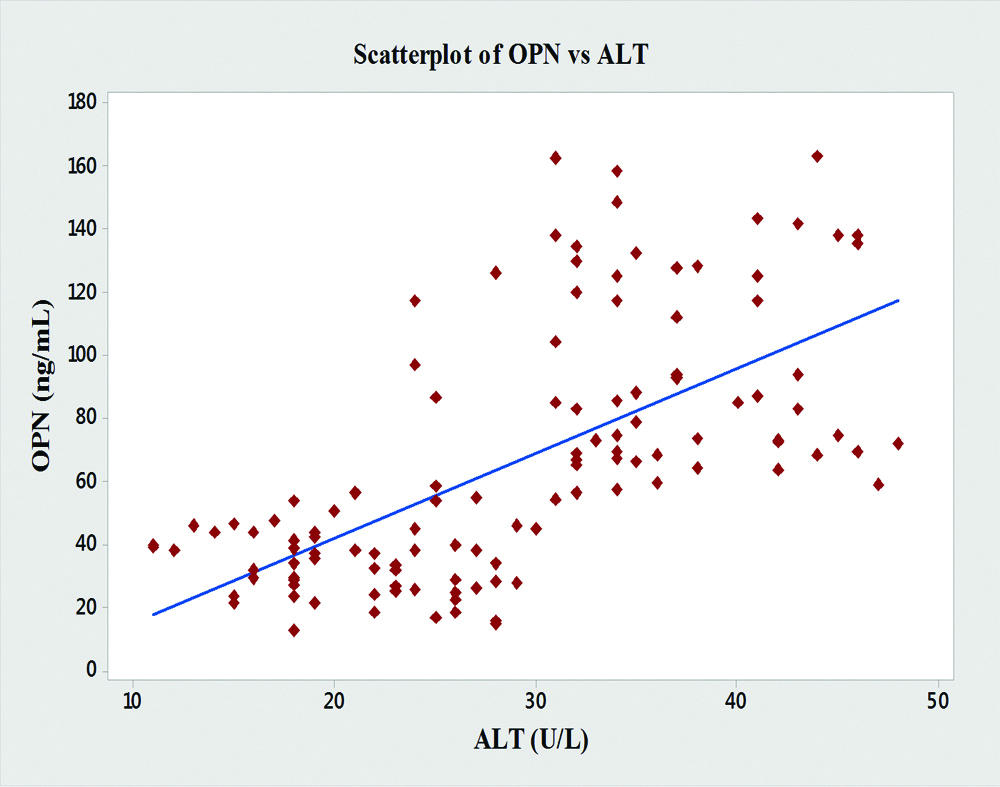
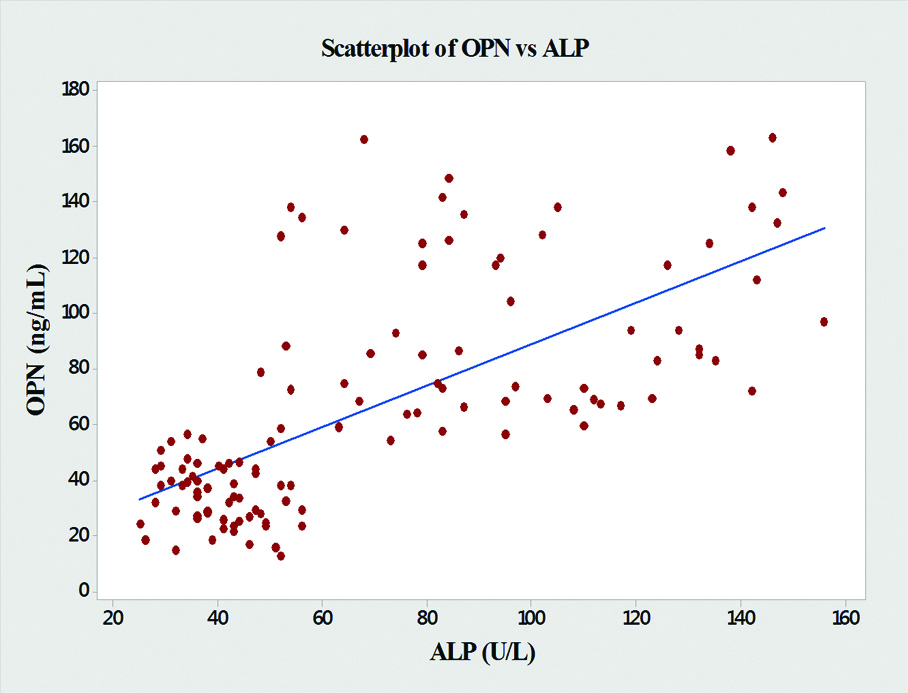
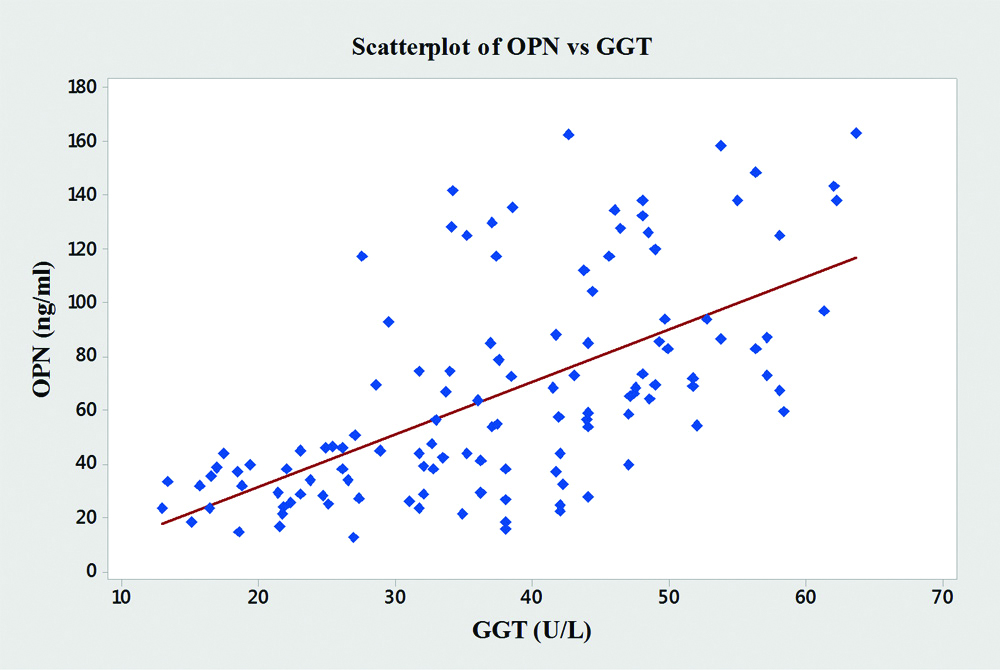
In the present study, significant correlations between the level of OPN and the liver enzymes (AST, ALT and ALP) were found [Table/Fig-4]. OPN showed significant positive correlations with AST (r=0.76, p<0.001), ALT (r=0.64, p<0.001), ALP (r=0.68, p<0.001) and GGT (r=0.61, p<0.001) [Table/Fig-5,6,7 and 8].
Pearson’s correlation coefficients of OPN with Liver enzymes.
| Variables | Pearson’s correlation coefficient of OPN with variables |
|---|
| r | p-value |
|---|
| AST | 0.76 | <0.001 |
| ALT | 0.64 | <0.001 |
| ALP | 0.68 | <0.001 |
| GGT | 0.61 | <0.001 |
<0.05 statistically significant
AST: Serum aspartate transaminases; ALT: Alanine transaminases; ALP: Alkaline phosphatase; GGT: Gamma glutamyl transferase; OPN: Serum osteopontin
Graph showing pearson’s correlations of OPN and AST of the subjects.
Graph showing pearson’s correlations of OPN and ALT of the subjects.
Graph showing pearson’s correlations of OPN and ALP of the subjects.
Graph showing pearson’s correlations of OPN and GGT of the subjects.
Upon ROC curve analysis, OPN had the maximum area under curve as compared to GGT and AST/ALT ratio [Table/Fig-9,10].
ROC curve of OPN, GGT and AST/ALT ratio.
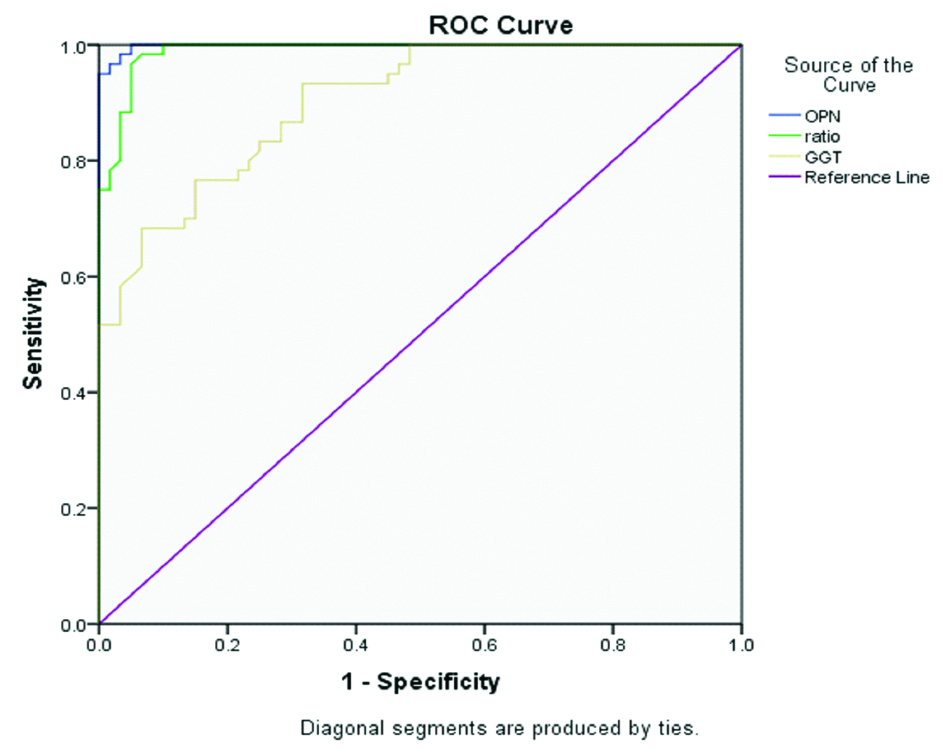
Table showing area under curve of OPN, GGT and AST/ALT ratio.
| Variable(s) | Area under curve | p value | 95% Confidence interval |
|---|
| Lower bound | Upper bound |
|---|
| OPN | 0.998 | <0.001 | 0.995 | 1.000 |
| AST/ALT ratio | 0.989 | <0.001 | 0.977 | 1.000 |
| GGT | 0.902 | <0.001 | 0.851 | 0.953 |
<0.05 statistically significant
AST: Serum aspartate transaminases; ALT: Alanine transaminases; GGT: Gamma glutamyl transferase; OPN: Serum osteopontin
Discussion
In the present study, clinically diagnosed cases of ALD (N=60) and healthy controls (N=60) were included. In the study, liver enzymes such as AST, ALT, ALP and GGT were significantly increased in the patients with ALD when compared to the healthy control subjects (p<0.001). Similar findings were observed in various studies [19-30]. In the present study, AST/ALT ratio was significantly elevated in alcoholic patients when compared to the healthy controls (p<0.001). This finding is in consistent with the findings of some studies which concluded that AST/ALT ratio was good indicator of cirrhosis in ALD [31-33]. The AST/ALT ratio is not a sensitive and specific marker of ALD especially in the advanced stages. On the other hand, GGT which is a membrane bound glycoprotein enzymeis widely used marker for excessive and repeated alcohol intake. The cause of increased serum GGT in chronic alcoholics is due to induction of hepatic microsomal enzymes. The serum GGT alone is not specific for ALD because it is raised in other conditions. Therefore, the authors wanted to determine the role of serum OPN as a biomarker of severity of ALD as several studies suggested that the serum OPN correlated with degree of fibrosis in chronic alcoholics [34,35].
In the study, significant increase was observed in the level of OPN in the patients with ALD when compared with the control subjects (p<0.001). This is in consistence with the findings of a number of other studies. [14-17,36-38]. In the present study, significant associations between the level of OPN and the liver enzymes (AST, ALT, ALP) was observed. OPN showed significant positive correlations with AST, ALT, ALP and GGT [Table/Fig-4,5,6,7 and 8]. Similar findings were shown by Patauraux S et al., who suggested that serum OPN levels correlated with stage of fibrosis and hepatic inflammation and Seth D et al., who also showed that OPN expression positively correlated with the severity of ALD [16,17]. Furthermore, Zhao L et al., also found that plasma OPN levels are predictive of cirrhosis in Hepatitis B virus (HBV) infection patients. Thus, they showed that it might be used as a marker evaluating the risk of cirrhosis in patients with HBV infection [37].
The area under curve obtained in the ROC curve for plasma OPN was highest as compared to GGT and AST/ALT ratio. This shows that OPN is a better predictor of ALD as compared to both other markers (p<0.001). Patauraux S et al., also showed that serum OPN level, blood test algorithm FibroMeter®, fibrosis hyaluronate predicted fibrosis with same accuracy and the serum OPN levels accurately estimated advanced fibrosis (fibrosis stage ≥3) with area under ROC = 0.91 (0.83, 0.95) and cirrhosis (Fibrosis stage = 4) with area under ROC = 0.91 (0.80, 0.96) in alcoholic patients [16,37]. Moreover, Ge X et al., suggested a protective role of OPN and suggested that OPN can protect from early alcohol-induced liver injury by blocking the gut-derived Lipopolysaccharide (LPS) and Tumour Necrosis Factor α (TNFα) effects the liver, thus, decreasing the progression of alcoholic hepatitis [39]. On the other hand, a study proposed that milk OPN can block OPN mediated pathways and can guard against hepatic inflammation and steatosis through their gut protective effects [40]. In addition to this, Yang L et al., developed fluorescent nanoprobe which images mIR-155 and OPN mRNA and images the evolution from alcoholic fatty liver to steatohepatitis, thus can monitor the effect of therapeutic strategy in reversing alcohol caused liver damage [41]. Thus, treatments targeting OPN can be further be explored in order to discover full potential of its therapeutic ramifications.
Limitation(s)
Due to time constraints and limited number of cases available, a convenient sample size of 120 was selected. Therefore, small sample size was a limitation of the study.
Conclusion(s)
Serum OPN can serve as a biomarker in the diagnosis of ALD and is a better predictor of ALD as compared to GGT and AST/ALT ratio. Validation of novel biomarkers such as OPN will help in establishing new diagnostic and prognostic blood tests algorithms having higher sensitivity and specificity in diagnosis of ALD. Further studies on a larger sample size are required for indepth evaluation of the possible role of Serum OPN as biomarkers of ALD. This pilot study needs to be substantiated with a larger sample size with different ethnicities.
[1]. Asrani SK, Devarbhavi H, Eaton J, Kamath PS, Burden of liver diseases in the worldJ Hepatol 2019 70(1):151-71.10.1016/j.jhep.2018.09.01430266282 [Google Scholar] [CrossRef] [PubMed]
[2]. Mukherjee PS, Vishnubhatla S, Amarapurkar DN, Das K, Sood A, Chawla YK, Etiology and mode of presentation of chronic liver diseases in India: A multi centric studyPLoS One 2017 12(10):e0187033Published 2017 Oct 2610.1371/journal.pone.018703329073197 [Google Scholar] [CrossRef] [PubMed]
[3]. Public health problems caused by harmful use of alcohol [Internet]. Apps.who.int. 2005 [cited 9 June 2020]. Available from: https://apps.who.int/gb/archive/pdf_files/WHA58/A58_18-en.pdf [Google Scholar]
[4]. Burra P, Senzolo M, Adam R, Delvart V, Karam V, Germani G, Liver transplantation for alcoholic liver disease in Europe: A study from the ELTR (European Liver Transplant Registry)Am J Transplant 2010 10:138-48.10.1111/j.1600-6143.2009.02869.x19951276 [Google Scholar] [CrossRef] [PubMed]
[5]. Ishak KG, Zimmerman HJ, Ray MB, Alcoholic liver disease: Pathologic, pathogenetic and clinical aspectsAlcohol Clin Exp Res 1991 15(1):45-66.10.1111/j.1530-0277.1991.tb00518.x2059245 [Google Scholar] [CrossRef] [PubMed]
[6]. Menon KV, Gores GJ, Shah VH, Pathogenesis, diagnosis, and treatment of alcoholic liver diseaseMayo Clin Proc 2001 76(10):1021-29.10.4065/76.10.102111605686 [Google Scholar] [CrossRef] [PubMed]
[7]. Gao B, Bataller R, Alcoholic liver disease: Pathogenesis and new therapeutic targetsGastroenterology 2011 141(5):1572-85.10.1053/j.gastro.2011.09.00221920463 [Google Scholar] [CrossRef] [PubMed]
[8]. Setshedi M, Wands JR, Monte SM, Acetaldehyde adducts in alcoholic liver diseaseOxid Med Cell Longev 2010 3:178-85.10.4161/oxim.3.3.1228820716942 [Google Scholar] [CrossRef] [PubMed]
[9]. FarfánLabonne BE, Gutiérrez M, Gómez-Quiroz LE, Konigsberg Fainstein M, Bucio L, Souza V, Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damageCell Biol Toxicol 2009 25:599-609.10.1007/s10565-008-9115-519137438 [Google Scholar] [CrossRef] [PubMed]
[10]. Parker R, Kim SJ, Gao B, Alcohol, adipose tissue and liver disease: Mechanistic links and clinical considerationsNat Rev Gastroenterol Hepatol 2018 15(1):50-59.10.1038/nrgastro.2017.11628930290 [Google Scholar] [CrossRef] [PubMed]
[11]. Thapa BR, Walia A, Liver function tests and their interpretationIndian J Pediatr 2007 74(7):663-71.10.1007/s12098-007-0118-717699976 [Google Scholar] [CrossRef] [PubMed]
[12]. Mauro P, Renze B, Wouter W, In: Tietz text book of clinical chemistry and molecular diagnostics4th editionCarl AB, Edward R, David EB, editors. Elsevier; 2006. Enzymes; pp. 604-616 [Google Scholar]
[13]. Puukka K, Hietala J, Koivisto H, Anttila P, Bloigu R, Niemelä O, Additive effects of moderate drinking and obesity on serum gamma-glutamyl transferase activityAm J Clin Nutr 2006 83:1351-54.10.1093/ajcn/83.6.135116789344 [Google Scholar] [CrossRef] [PubMed]
[14]. Wen Y, Jeong S, Xia Q, Kong X, Role of osteopontin in liver diseasesInternational Journal of Biological Sciences 2016 12(9):112110.7150/ijbs.164452757048627570486 [Google Scholar] [CrossRef] [PubMed] [PubMed]
[15]. Morales-Ibanez O, Domínguez M, Ki SH, Marcos M, Chaves JF, Nguyen-Khac E, Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitisHepatology 2013 58(5):1742-56.10.1002/hep.2652123729174 [Google Scholar] [CrossRef] [PubMed]
[16]. Patouraux S, Bonnafous S, Voican CS, Anty R, Saint-Paul MC, Rosenthal-Allieri MA, The osteopontin level in liver, adipose tissue and serum is correlated with fibrosis in patients with alcoholic liver diseasePLoS One 2012 7(4):e3561210.1371/journal.pone.003561222530059 [Google Scholar] [CrossRef] [PubMed]
[17]. Seth D, Duly A, Kuo PC, McCaughan GW, Haber PS, Osteopontin is an important mediator of alcoholic liver disease via hepatic stellate cell activationWorld J Gastroenterol 2014 20(36):13088-104.10.3748/wjg.v20.i36.1308825278703 [Google Scholar] [CrossRef] [PubMed]
[18]. Mandayam S, Jamal MM, Morgan TR, Epidemiology of alcoholic liver diseaseSemin Liver Dis 2004 24:217-32.10.1055/s-2004-83293615349801 [Google Scholar] [CrossRef] [PubMed]
[19]. Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study GroupGut 1997 41:845-50.10.1136/gut.41.6.8459462221 [Google Scholar] [CrossRef] [PubMed]
[20]. Magdaleno F, Ge X, Fey H, Lu Y, Gaskell H, Blajszczak CC, Osteopontin deletion drives hematopoietic stem cell mobilization to the liver and increases hepatic iron contributing to alcoholic liver diseaseHepatology Communications 2018 2(1):84-98.10.1002/hep4.111629404515 [Google Scholar] [CrossRef] [PubMed]
[21]. Savolainen VT, Liesto K, Männikkö A, Penttilä A, Karhunen PJ, Alcohol consumption and alcoholic liver disease: Evidence of a threshold level of effects of ethanolAlcoholism: Clinical and Experimental Research 1993 17(5):1112-17.10.1111/j.1530-0277.1993.tb05673.x8279675 [Google Scholar] [CrossRef] [PubMed]
[22]. Sebastiani G, Alberti A, Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsyWorld Journal of Gastroenterology: WJG 2006 12(23):368210.3748/wjg.v12.i23.368216773685 [Google Scholar] [CrossRef] [PubMed]
[23]. Das SK, Vasudevan DM, Biochemical diagnosis of alcoholismIndian Journal of Clinical Biochemistry 2005 20(1):35-42.10.1007/BF0289303923105491 [Google Scholar] [CrossRef] [PubMed]
[24]. Maithreyi R, Janani AV, Krishna R, Shweta A, Edwin RR, Mohan SK, Erythrocyte lipid peroxidation and antioxidants in chronic alcoholics with alcoholic liver diseaseAsian J Pharm Clin Res 2010 3:183-85. [Google Scholar]
[25]. Mirunalini S, Arulmozhi V, Arulmozhi T, Curative effect of garlic on alcoholic liver diseased patientsJordan Journal of Biological Sciences 2010 3(4):147-51. [Google Scholar]
[26]. Jang ES, Jeong SH, Hwang SH, Kim HY, Ahn SY, Lee J, Effects of coffee, smoking, and alcohol on liver function tests: A comprehensive cross-sectional studyBMC Gastroenterology 2012 12(1):14510.1186/1471-230X-12-14523075166 [Google Scholar] [CrossRef] [PubMed]
[27]. Al-Jumaily EF, The effect of chronic liver diseases on some biochemical parameters in patients serumCurrent Research Journal of Biological Sciences 2012 4(5):638-42. [Google Scholar]
[28]. Hyder MA, Hasan M, Mohieldein AH, Comparative levels of ALT, AST, ALP and GGT in liver associated diseasesEuro J Exp Bio 2013 3(2):280-84. [Google Scholar]
[29]. Gayathri B, Vasantha M, Comparative levels of liver enzymes in patients with various liver disordersInt J Pharm Bio Sci 2015 6(4):1099-102. [Google Scholar]
[30]. Brandl K, Hartmann P, Jih LJ, Pizzo DP, Argemi J, Ventura-Cots M, Dysregulation of serum bile acids and FGF19 in alcoholic hepatitisJournal of Hepatology 2018 69(2):396-405.10.1016/j.jhep.2018.03.03129654817 [Google Scholar] [CrossRef] [PubMed]
[31]. Nyblom H, Berggren U, Balldin J, Olsson R, High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinkingAlcohol & Alcoholism 2004 39(4):336-39.10.1093/alcalc/agh07415208167 [Google Scholar] [CrossRef] [PubMed]
[32]. Nyblom H, Bjornsson E, Simren M, Aldenborg F, Almer S, Olsson R, The AST/ALT ratio as an indicator of cirrhosis in patients with PBCLiver International 2006 26:840-45.10.1111/j.1478-3231.2006.01304.x16911467 [Google Scholar] [CrossRef] [PubMed]
[33]. Whitfield JB, Masson S, Liangpunsakul S, Hyman J, Mueller S, Aithal G, Evaluation of laboratory tests for cirrhosis and for alcohol use, in the context of alcoholic cirrhosisAlcohol 2018 66:01-07.10.1016/j.alcohol.2017.07.00629277282 [Google Scholar] [CrossRef] [PubMed]
[34]. Bruha R, Vitek L, Smid V, Osteopontin-A potential biomarker of advanced liver diseaseAnnals of Hepatology 2020 19(4):344-52.10.1016/j.aohep.2020.01.00132005637 [Google Scholar] [CrossRef] [PubMed]
[35]. Matsue Y, Tsutsumi M, Hayashi N, Saito T, Tsuchishima M, Toshikuni N, Serum osteopontin predicts degree of hepatic fibrosis and serves as a biomarker in patients with hepatitis C virus infectionPloS One 2015 10(3):e011874410.1371/journal.pone.011874425760884 [Google Scholar] [CrossRef] [PubMed]
[36]. Arai M, Yokosuka O, Kanda T, Fukai K, Imazeki F, Muramatsu M, Serum osteopontin levels in patients with acute liver dysfunctionScandinavian Journal of Gastroenterology 2006 41(1):102-10.10.1080/0036552051002406116373283 [Google Scholar] [CrossRef] [PubMed]
[37]. Zhao L, Li T, Wang Y, Pan Y, Ning H, Hui X, Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infectionInternational Journal of Clinical Practice 2008 62(7):1056-62.10.1111/j.1742-1241.2007.01368.x17537188 [Google Scholar] [CrossRef] [PubMed]
[38]. Fouad SA, Ghaffar Mohamed NA, Fawzy MW, Moustafa DA, Plasma osteopontin level in chronic liver disease and hepatocellular carcinomaHepat Mon 2015 15(9):e3075310.5812/hepatmon.3075326500684 [Google Scholar] [CrossRef] [PubMed]
[39]. Ge X, Leung TM, Arriazu E, Lu Y, Urtasun R, Christensen B, Osteopontin binding to lipopolysaccharide lowers tumor necrosis factor-α and prevents early alcohol-induced liver injury in miceHepatology 2014 59(4):1600-16.10.1002/hep.2693124214181 [Google Scholar] [CrossRef] [PubMed]
[40]. Ge X, Lu Y, Leung TM, Sørensen ES, Nieto N, Milk osteopontin, a nutritional approach to prevent alcohol-induced liver injuryAmerican Journal of Physiology-Gastrointestinal and Liver Physiology 2013 304(10):G929-39.10.1152/ajpgi.00014.201323518682 [Google Scholar] [CrossRef] [PubMed]
[41]. Yang L, Chen Y, Pan W, Wang H, Li N, Tang B, Visualizing the conversion process of alcohol-induced fatty liver to steatohepatitis in vivo with a fluorescent nanoprobeAnal Chem 2017 89(11):6196-201.10.1021/acs.analchem.7b0114428492308 [Google Scholar] [CrossRef] [PubMed]