Metabolic syndrome, or X syndrome, is a set of metabolic abnormalities that include: high blood pressure, high blood sugar, abdominal obesity, abnormal cholesterol and Triglyceride (TG) levels, taken together, these factors increase the risk of cardiovascular disease, diabetes (type 2) and stroke and myocardial infarction [1-4]. Cardiac Ischemia is highly prevalent in these patients because of insufficient blood supply to the heart tissue and due to congestion of the arteries [5].
Recent studies indicate a widespread prevalence of metabolic syndrome worldwide, including Iran [6], with 42% of women and 24% of men suffering from this syndrome [1,7]. Today, people with metabolic syndrome are three times more likely to develop heart diseases [1]. Abdominal obesity and insulin resistance are components of the metabolic syndrome that may increase blood pressure, hyperglycaemia, lower levels of HDL and raise serum cholesterol and increase the risk of cardiovascular diseases [1,8,9]. Insulin resistance also increases the release of lipoprotein rich TGs into the bloodstream [10,11].
Omega-3 is a long chain Polyunsaturated Fatty Acid (PUFA) composed of Eicosapentaenoic Acid (EPA) (C20: 5n-3), Docosahexaenoic Acid (DHA) (C22: 6n-3) and Alpha-Linolenic Acid (ALA) (C18: 3n-3) [12,13]. EPA, DHA and ALA need to reach the body through nutrients or supplements as they are produced in very small quantities within the body [12,14]. Omega-3 fatty acid improves endothelial status by ascorbic acid adherence to membrane phospholipid, and since metabolic syndrome disrupts the endothelial system, it can be used as an endothelial enhancer in individuals with metabolic syndrome and heart diseases [15]. One study showed, there is a direct relationship between low levels of omega-3 fatty acids in the blood and sudden heart attacks, and high levels of this fatty acid in the serum may decrease myocardial infarction [16].
Since, the results of studies focusing on the effect of omega 3 supplementation on indicators of metabolic syndrome such as TG level, weight, blood pressure and FBS in ischemic heart diseases patients are inconclusive [11,17-19].
In this study, primary outcome was to evaluate the effect of omega-3 fatty acids in Ischemic Heart Disease patients. Secondary outcome was to evaluate the effect of omega-3 fatty acids on TG level and lipid profile, weight, blood pressure and FBS.
Materials and Methods
Study Design and Participants
This study was a double-blind randomised, placebo-controlled trial. The project was approved on 7/31/2019 by the Research Ethics Committee of Urmia University of Medical Sciences (Ethics Code: IR.UMSU.REC.1398.190). This study was also registered at Iranian Registry of Clinical Trials (www.irct.ir) in November 2019 (IRCT Code: IRCT20190819044563N1). The study was performed at the Sayed al-Shohada Heart Training Medical Center in Urmia, Iran and the project was conducted between December 2019 and March 2020. The study was conducted in accordance with the Helsinki Declaration and written informed consent was obtained from all participants before the study.
Sample size as based on TG means within the study by Oh PC et al., with 95% confidence interval and 90% test power, and with a 10% probability of falling, 45 patients in each group (intervention and placebo) was determined [20]. Ninety men and women with a mean age of 53.6±9.7 (mean±SD) years were included in the study according to inclusion and, exclusion criteria and, simple sampling method [Table/Fig-1].
Flow chart of Randomised Control Trial of omega 3 supplementation in patients with metabolic syndrome and ischemic heart disease.
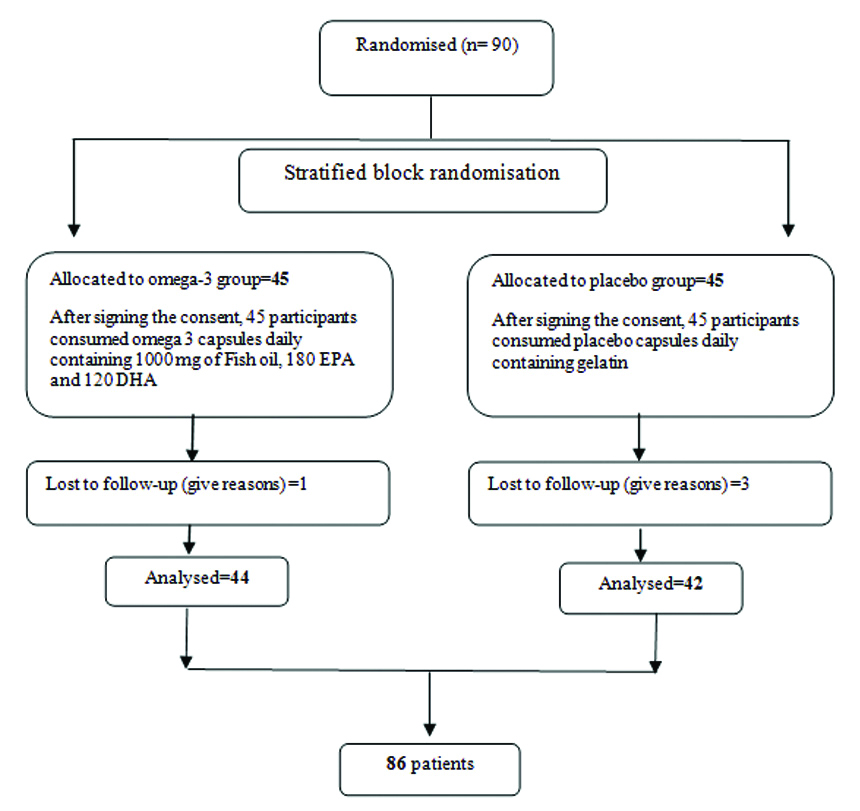
Inclusion criteria were defined as having a history of cardiac ischemia and metabolic syndrome. Metabolic syndrome as defined by the National Diabetes Foundation (IDF) includes waist circumference ≥80 cm for women and ≥94 cm for men, with at least two cases of the following: FBS ≥100 mg/dL or drug treatment for this disorder, systolic blood pressure ≥130 and diastolic blood pressure ≥85 mm Hg, HDL <50 mg/dL for women and HDL <40 mg/dL for men and TGs ≥150 mg/dL or drug treatment for these lipid abnormalities [21]. Cardiac Ischemia consists of a lack of adequate blood supply to the heart tissue or obstruction of the arteries [5] has been recognised by a specialist Cardiologist based on non-invasive tests such as echocardiography and exercise tests, invasive tests including coronary angiography and the administration of anti-ischemic drugs [22]. Exclusion criteria were mental, emotional, cognitive disorders, autoimmune diseases, taking corticosteroids, having cancer, alcohol consumption, pregnancy, lactation, infectious diseases, chronic kidney disease, chronic and acute liver diseases, fish allergies, drug abuse, consumption of more than one serving of fish per week and using anticoagulants such as warfarin.
Participants hospitalised at the Sayyed al-Shohada Medical Center were randomly assigned to the study between November and December 2019 according to inclusion, and exclusion criteria. Patients were fully informed about aim of the study with informed consents before the study began. Participants were randomly divided into two groups-intervention and placebo according to stratified block randomisation method and consistent with gender and BMI. Omega-3 and placebo supplements were coded by a third party who was not in the study. The packaging of both omega-3 and placebo capsules were similar in shape, size, and weight. Both the researchers and the participants were not aware of any type of capsules utilised in each group (placebo or omega-3). By telephone, people were asked to visit the Cardiovascular Clinic of Sayed al-Shohada Heart Training Medical Center on a particular day and hour.
All subjects were also asked to fast for 12 hours. A 5 cc blood sample was collected for FBS testing, cholesterol, TG, LDL, HDL. In addition, participants completed demographic, food frequency, physical activity, and 24-hour recall questionnaires. The intervention group was given a daily omega-3 fatty acid capsule containing 1000 mg of fish oil, 180 mg of EPA and 120 mg of DHA (manufactured at NUTRALab Canada plant and packaged at Zahravi-Iran Tabriz Pharmaceutical Company). The placebo group also received daily one capsule of gelatin (manufactured at Sobhan Pharmaceutical Company) that was similar to the original form. Supplements were given monthly for only 30 days, and after each month, the follow-up visit was completed, and after the blank packets were delivered, the supplement was given for the following month. Participants were asked not to change their usual physical activity and diet during the study. The intervention period was 12 weeks.
Assessment
Height, weight and waist circumference were measured by a trained nutritionist in the first, six, and 12 weeks [12]. Height and weight of subjects with minimal clothing and no shoes were measured using Rasa scales and gauges with a maximum error of ±0.1 kg for weight and accuracy of 0.1 m for height. BMI was obtained by using height and weight (Kg) division by squared height (m2). The waist circumference of the upper part of the iliac bone was also measured from a light-weight, parallel to the bottom surface, following an exhalation and using a flexible plastic tape meter with no pressure and 0.1 cm accuracy.
Blood samples were taken at the start, and at the end of the study, after a 12-hour fast between 8:00 am and 10:00 am while the patient was fasting and in sitting position. Five cc venous blood were taken from all patients. The serum was then centrifuged at 4000 rpm for seven minutes and stored at -70°C until analysis. Biochemical parameters were analysed for FBS, HDL, LDL, TG and cholesterol concentrations. FBS concentrations were measured by glucose oxidase and HDL, LDL, TG, and cholesterol enzymatically using Pars test kits (Made in Iran) and Pars test (FBS98007 series, cholesterol 98001 series, LDL 98005 series, HDL 98004 series, TG97006) made in Iran.
Blood pressure was measured by a specialist Cardiologist with calibrated digital blood pressure monitor (SANITAS, Germany) and after a 5-minute rest, before and after the intervention.
A 24-hour dietary intake questionnaire was administered during the primary and 12 weeks of the study and each time for three days (two working days and one day-off) to receive baseline dietary intake to make sure that dietary habits remained unchanged, moreover to assess omega-3 intake. The food information obtained from the 24-hour recall questionnaire entered into the nutritionist-4 program (version 2. 5.3). To assess reliability, the nutritional status of dietary intake of omega-3s, a validated food frequency questionnaire [23] was evaluated within the first and 12 weeks. This questionnaire was filled by a trained nutritionist, then the knowledge was converted to gram according to the diet charts for Iran. Using this method, the omega-3 intake of food was studied and, compared with the results of dietary 24-recall. International Physical Activity Level Questionnaire (IPAQ) was used to compare the physical activity levels within the primary and 12 weeks [24] and the IPAQ data were reported in the tables as MET.
Statistical Analysis
Quantitative variables are reported as mean±SD and qualitative variables are reported as percentages. Data were analysed using Kolmogorov-Smirnov test. To compare the mean of normal variables (age, FBS, cholesterol, TG, HDL, LDL, waist circumference, systolic blood pressure, food intakes, such as consumed meat, fat and omega-3 intake) at baseline and their changes in the 2 groups were used Independent T-test. Mann Whitney U test was also used to compare the mean of variables that did not have a normal distribution (carbohydrate, fruit, vegetable, simple sugar, dairy, diastolic blood pressure, and weight). Chi-square test was used to compare the frequency of qualitative variables (sex, place of residence). To compare within groups (before and after) in each group Paired t-test was used for normal variables and the Wilcoxon test, if data were not normal. Repeated Measures test was used to compare the mean of variables including weight, BMI, two waist circumference, systolic blood pressure and diastolic blood pressure (adjusted for baseline values for blood pressure). Data analysis was performed using Statistical Package for the Social Sciences (SPSS) 17 software and the significance level was considered less than 0.05 (p<0.05).
Results
Ninety people participated in this study and then 86 patients completed the study. The mean age of the patients within the intervention group was (55±9.6) years and therefore, the placebo group was (52.2±9.52) years, which was not significantly different (p=0.17). There were 22 men and 22 women in intervention group also, 21 men and 21 women in placebo group. Within the intervention group, 59.1% of the population lived in the city and 40.9% in the rural area, while within the placebo group 71.4% lived in the city and 28.6% lived in the rural area, there was no statistically significant difference (p=0.24) between two groups. [Table/Fig-2] demonstrates the comparison of baseline variables between intervention and placebo groups.
Comparison of baseline variables between intervention and placebo groups.
| Variable | Intervention group Mean±SD | Placebo group Mean±SD | p-value |
|---|
| Age (year) | 55±9.6 | 52.12±9.52 | 0.17* |
| FBS (mg/dL) | 110.6±19.52 | 106.33±15.6 | 0.29* |
| Cholesterol (mg/dL) | 160.5±39.3 | 165.33±37.3 | 0.57* |
| TG (mg/dL) | 171.3±53.7 | 151.7±48.3 | 0.08* |
| HDL (mg/dL) | 38.2±6.7 | 41.5±9.4 | 0.07* |
| LDL (mg/dL) | 83.7±24.4 | 94.0±24.9 | 0.07* |
| WC (cm) | 105.8±9.7 | 107.6±8.8 | 0.37* |
| BMI (Kg/m2) | 30.74±4.5 | 32.11±5.01 | 0.19* |
| Systolic blood pressure (mmHg) | 129.6±17.35 | 136.5±11.3 | 0.03* |
| Diastolic blood pressure (mmHg) | 81.82±9.02 | 83.74±14.6 | 0.17** |
| Weight (Kg) | 81.72±12.23 | 87.22±14.4 | 0.05** |
| MET (MET·min·wk−1) | 53.9±27.5 | 60.9±30.6 | 0.30** |
| Meat (Unit) | 4.6±1.06 | 5.6±1.4 | ≤0.001* |
| Fat (Unit) | 6.2±1.8 | 7.9±1.7 | ≤0.001* |
| CHO (Unit) | 10.5±2.5 | 9.2±3.2 | 0.030** |
| Fruit (Unit) | 2.6±1.6 | 2±1.2 | 0.07** |
| Vegetable (Unit) | 2.5±1.6 | 1.9±1.13 | 0.08** |
| Sugar (Unit) | 2.92±1.4 | 2.96±1.5 | 0.93** |
| Dairy (Unit) | 2.05±0.83 | 1.9±0.8 | 0.36** |
| Omega3 fatty acids (gram) | 6.05±3.82 | 6.45±2.74 | 0.58* |
*Independent T-Test; **Mann Whitney U test.
TG: Triglyceride; SD: Standard deviation; FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; WC: Waist circumference; BMI: Body mass index; Met: Metabolic equivalent of task; CHO: Carbohydrate
Based on the exclusion criteria, there were no smokers among participants. Finally, 86 patients completed the study [Table/Fig-1]. In the intervention group, daily intake of 180 mg EPA and 120 mg DHA omega-3s significantly decreased the mean weight (p<0.05). Changes in BMI values within the intervention group were -0.5 kg/m2 (p<0.001) but considering placebo group changes, BMI did not decrease significantly during the study (p=0.09) [Table/Fig-3]. TG values within the intervention group decreased significantly (p<0.001). Omega-3 intake also significantly (p=0.009) reduced the mean systolic blood pressure. FBS also showed a significant decrease (p<0.001), with a mean of -6.7 mg/dL within the intervention group. Moreover, the mean HDL within the intervention group significantly increased {+5.8 mg/dL (p<0.001)} [Table/Fig-4,5].
Comparing the mean of BMI, weight, waist circumference, systolic and diastolic blood pressure in intervention and placebo groups.
| Variables | Intervention groupMean±SD | Placebo groupMean±SD | p-trend |
|---|
| Baseline | Week 6 | p-value† | Week 12 | p-value†† | p-value* | Baseline | Week 6 | p-value† | Week 12 | p-value†† | p-value** |
|---|
| Weight (Kg) | 81.73±12.23 | 80.6±12 | <0.001 | 80.5±12 | 0.62 | <0.001 | 87.23±14.4 | 86.99±14.4 | 0.19 | 87.6±14.5 | <0.001 | 0.002 | 0.03 |
| BMI (Kg/m2) | 30.74±4.5 | 30.3±4.4 | <0.001 | 30.25±4.3 | 0.42 | <0.001 | 32.11±5.01 | 32.03±5.05 | 0.22 | 32.3±5.07 | <0.001 | 0.002 | 0.09 |
| WC (cm) | 105.8±9.7 | 103.9±9.05 | <0.001 | 103.25±8.4 | 0.11 | <0.001 | 107.5±8.8 | 107.9±9.3 | 0.51 | 108.9±9.3 | 0.001 | 0.001 | 0.06 |
| Systolic blood pressure (mmHg) | 129.6±17.34 | 127.9±16.5 | 0.39 | 119.5±27.72 | 0.03 | 0.009 | 136.5±11.3 | 138.9±15.1 | 0.17 | 138.5±16.08 | 0.82 | 0.38 | <0.001 |
| Diastolic blood pressure (mmHg) | 81.82±9.03 | 81.84±10.2 | 0.99 | 80.32±11.33 | 0.22 | 0.6 | 83.74±14.7 | 84±15.5 | 0.74 | 83.5±16.3 | 0.49 | 0.74 | 0.4 |
p-value†=Changes between week 6 and baseline, p-value††=Changes between week 12 and 6, p-value*=Changes within intervention group
p-value**=Changes within placebo group, P-trend=Changes between placebo and intervention group.
Abbreviations: BMI: body mass index, WC: Waist circumference; SD: Standard deviation
Comparing the variables between intervention and placebo groups.
| Intervention groupMean±SD | Placebo groupMean±SD |
|---|
| Variables | Baseline | Week 12 | p-value | Baseline | Week 12 | p-value |
|---|
| FBS (mg/dL) | 110.52±19.7 | 103.81±16.6 | <0.001* | 106.9±14 | 103.8±14.6 | 0.11* |
| Cholesterol (mg/dL) | 160.51±39.3 | 156.6±39.5 | 0.25* | 165.33±37.23 | 163.33±41.05 | 0.67* |
| TG (mg/dL) | 171.21±53.7 | 153.05±42.2 | <0.001* | 151.73±49.6 | 164.73±45.5 | 0.08* |
| HDL (mg/dL) | 38.05±6.7 | 43.9±9 | <0.001* | 41.15±9.3 | 40.5±9 | 0.58* |
| LDL (mg/dL) | 83.7±24.4 | 83.4±23.8 | 0.87* | 94±25 | 91.9±25.2 | 0.04* |
| MET (MET min wk-1) | 53.9±27.5 | 50.8±27.9 | 0.05** | 60.9±30.5 | 58.7±30.6 | 0.01** |
| CHO (Unit) | 10.5±2.5 | 10.4±2.5 | 0.10* | 9.15±3.12 | 9.05±3.2 | 0.15* |
| Fruit (Unit) | 2.51±1.6 | 2.5±1.52 | 0.41* | 1.98±1.12 | 1.9±1.1 | 0.18* |
| Vegetable (unit) | 2.42±1.6 | 2.47±1.5 | 0.20** | 1.9±1.3 | 1.8±1.2 | 0.06** |
| Sugar (Unit) | 2.9±1.4 | 2.7±1.1 | 0.01* | 2.96±1.5 | 2.9±1.4 | 0.25* |
| Meat (Unit) | 4.54±1.05 | 4.5±1.06 | 0.29* | 5.6±1.4 | 5.53±1.4 | 0.06* |
| Dairy (Unit) | 2.1±0.9 | 2.2±0.8 | 0.26** | 1.92±0.78 | 1.9±0.71 | 0.4** |
| Fat (Unit) | 6.3±1.9 | 6.1±1.9 | 0.07** | 7.93±1.7 | 7.95±1.6 | 0.6** |
| Omega 3 fatty acids (gram) | 6.05±3.8 | 5.2±3.4 | <0.001* | 6.4±2.74 | 5.7±2.21 | <0.001* |
*Paired t-test, **Wilcoxon test
FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; MET: Metabolic equivalent of task; CHO: Carbohydrate; TG: Triglyceride; SD: Standard deviation
Comparison of mean changes of variables between intervention and placebo groups.
| Variables | Intervention group Mean±SE | Placebo group Mean±SE | p-value |
|---|
| FBS (mg/dL) | -6.71±1.5 | -2.13±1.3 | 0.02 |
| Cholesterol (mg/dL) | -4±3.4 | -2±4.7 | 0.73 |
| TG (mg/dL) | -18.2±4.7 | 13±7.32 | <0.001 |
| HDL (mg/dL) | 5.8±0.9 | -0.7±1.2 | <0.001 |
| LDL (mg/dL) | -0.3±2.07 | -2.1±0.99 | 0.44 |
| MET (MET·min·wk-1) | -3.13±1.51 | -2.32±0.91 | 0.93 |
| Fruit (Unit) | -0.04±0.05 | -0.08±0.06 | 0.68 |
| Vegetables (Unit) | -0.1±0.08 | -.0.08±0.05 | 0.5 |
| Sugar (Unit) | -0.3±0.1 | -0.0±0.08 | 0.08 |
| Dairy (Unit) | 0.05±0.04 | -0.05±0.06 | 0.1 |
| CHO (Unit) | -0.09±0.05 | -0.09±0.06 | 0.88 |
| Meat (Unit) | -0.9±0.05 | -0.6±0.05 | 0.8 |
| Fat (Unit) | -0.09±0.05 | 0.04±0.05 | 0.033 |
| Omega3 fatty acids (gram) | -0.8±0.21 | -0.76±0.15 | 0.88 |
FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; MET: Metabolic equivalent of task; CHO: Carbohydrate; TG: Triglyceride; SE: Standard error
The mean waist circumference decreased from 105.8±9.7 cm to 103.25±8.4 cm at the end of the intervention within the intervention group (p<0.001) but considering to placebo group changes, waist circumference did not decrease significantly during the study (p=0.06). Also, LDL values within the intervention group did not decrease significantly (p=0.8). [Table/Fig-3,4 and 5] examines the comparisons between the two groups over 12 weeks.
All consumed food items containing omega 3 fatty acids were taken into account in calculation of total omega 3. There was a statistically difference between two groups at baseline regarding the consumption of meat, fat and carbohydrates, but their effects were eliminated by the analysis of co-variance.
Discussion
Factors that cause metabolic syndrome are factors which will increase the risk of cardiovascular disease, including high blood cholesterol, high waist circumference, high blood pressure, high blood sugar and high TGs [1,8,9]. This study showed that daily intake of 1000 mg of fish oil, 180 mg of EPA and 120 mg of DHA significantly decreased serum TG levels, weight, systolic blood pressure, FBS and increased HDL level (all p<0.05). However, there have been no significant changes in cholesterol, BMI, LDL, waist circumference and diastolic blood pressure.
Serum TGs within the intervention group decreased (171.21±53.7 vs 153.05±42.2 mg/dL p<0.001) after 12 weeks of omega-3 intake. This conclusion was consistent with previous studies [12,25,26]. A systematic review study examining the effect of EPA and DHA in 17 clinical trial articles on metabolic syndrome showed that serum TG levels decreased with the utilisation of omega-3 supplements [11]. This decrease in serum TG contains a variety of reasons, including the inhibition of two key acetyltransferase diacylglycerol and phosphatidic acid phosphohidrolase, both of which are involved in hepatic TG biosynthesis [27].
BMI was decreased within the intervention group (p<0.001), but there was no significant change during the study. This finding was also obtained in previous studies [17,18]. Contrary to the findings, in a similar study, participants consumed 900 mg/day for 24 weeks, BMI decrease significantly in subjects, which might be because of a long study period [26].
Serum HDL levels after 12 weeks of omega-3 fatty acid supplementation significantly increased (5.8 mg/dL) within the present study. This finding was also reported by Garcia-Lopez S et al., during a study on the effect of omega-3 supplements on metabolic syndrome indices [28]. But another study that supplemented omega-3 for healthy subjects for four weeks found no effect on serum HDL, possibly because of low study duration (4 weeks) and low sample size (n=57) [18]. Mejia-Barradas CM et al., examined the effect of omega-3 supplementation on the expression of Peroxisome Proliferation Activated Receptor Alpha (PPARα) and gamma in the subcutaneous tissue of obese adolescents for 12 weeks, showed that PPARα gene values increased which can be one of the causes of lower TGs, BMI within intervention group, weight, and increased HDL [29].
FBS levels were also significantly reduced. This result was the same as previous studies using omega-3s [28,30]. Systolic blood pressure decreased significantly within the intervention group (p<0.009), but no significant changes in diastolic blood pressure (p=0.4) were observed. This finding was in line with the findings of similar studies [26,28].
Cholesterol values within the present study did not change significantly (p=0.73). In line with other similar studies, including a meta-analysis that examined the results of 27 cross-sectional and case-control studies, no significant relationship was found between omega-3 intake, and serum cholesterol [31].
In the present study, there was no significant change in serum LDL within the intervention group, but within the placebo group serum LDL levels decreased by -2.1 mg/dL, however, LDL changes during this study were not statistically significant (p=0.44). This is similar to a systematic review and meta-analysis study examining the utilisation of omega-3 plus statins on serum LDL, with no significant change in serum LDL levels within the intervention group [31]. However, during a review study after examining the utilisation of this supplement alone on LDL values, Weintraub H found that serum LDL values increased [32].
One of the strengths of the present study is the inclusion of patients with metabolic syndrome and improved myocardial ischemia since metabolic syndrome can worsen the disease.
Limitation(s)
One of the limitations of this study was that inflammatory biomarkers like C-reactive protein or cardiac biomarkers were not studied to better demonstrate the effects of omega-3 fatty acids on inflammatory in subjects with metabolic syndrome and, the sample size used in this study could have been more.
Conclusion(s)
Omega-3 supplementation at 180 mg EPA and 120 mg DHA in patients with ischemic heart disease for 12 weeks improves some indicators of metabolic syndrome like serum TG, HDL (cardiovascular risk factors), FBS, weight, systolic blood pressure, and results in decreased risk of future coronary and ischemic heart diseases. In the present study, omega-3 fatty acid does not have any effect on cholesterol, LDL, BMI, waist circumference and diastolic blood pressure. Considering the beneficial effects of omega-3s supplements on inflammatory status in patients with metabolic syndrome, it is recommended to perform the study by assessing inflammatory markers like C-reactive protein and cardiac biomarkers in the future.
*Independent T-Test; **Mann Whitney U test.
TG: Triglyceride; SD: Standard deviation; FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; WC: Waist circumference; BMI: Body mass index; Met: Metabolic equivalent of task; CHO: Carbohydrate
p-value†=Changes between week 6 and baseline, p-value††=Changes between week 12 and 6, p-value*=Changes within intervention group
p-value**=Changes within placebo group, P-trend=Changes between placebo and intervention group.
Abbreviations: BMI: body mass index, WC: Waist circumference; SD: Standard deviation
*Paired t-test, **Wilcoxon test
FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; MET: Metabolic equivalent of task; CHO: Carbohydrate; TG: Triglyceride; SD: Standard deviation
FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; MET: Metabolic equivalent of task; CHO: Carbohydrate; TG: Triglyceride; SE: Standard error