Cardiovascular diseases continue to be a major cause of mortality in India contributing to 27% of proportional mortality as per statistics of 2016 [1]. Many risk factors for MI have been extensively researched upon such as smoking and tobacco use, Diabetes Mellitus (DM), Hypertension (HTN), dyslipidemia, obesity, psychosocial stress, elevated homocysteine levels and sedentary lifestyle [2]. Iron is one of the most important trace elements in the human body. It can accept and donate electrons by exchanging between ferrous and ferric forms. This generates reactive oxygen species through Fenton and Haber-Weiss reactions and is implicated to cause oxidative damage. It is in consideration of this, that Iron was implicated to increase risk of cardiovascular disease by causing bio-membrane damage [3]. Studies by Menke A et al., and Rajapurkar MM et al., have shown that excess body iron stores are associated with higher incidence or worse prognosis of cardiovascular diseases [4,5]. However, the role of iron is still controversial. Kervienen H et al., studied the association between serum iron and chronic heart disease in a nested case-control study. The subjects with low iron were at an increased risk (OR=9.8; 95% CI: 3.9-24.4). The probability of increased coronary events correlated with a decrease in serum iron levels [6].
In a prospective nested case-referent study, Ekblom K et al., the authors found an inverse risk association for MI in the highest quartiles of iron (OR=0.68; 95% CI: 0.48-0.96) and TS (OR=0.62; 95% CI: 0.42-0.89) in men. The researchers hence inferred that iron levels in the upper normal range seemed to be associated with a lower risk for first MI [7]. Inflammation has been identified as an important factor in Acute MI. It is caused by pro-inflammatory cytokines, which are released from the inflamed tissue by inflammatory and parenchymal cells. Hs-CRP is the classical acute phase reactant protein, the serum level of which has long been known to increase in Acute Myocardial Infarction (AMI) [8,9]. Hs-CRP is an attractive biomarker and has opsonising properties. It pushes the monocytes into the atheroma, suppresses the basal and induce nitric oxide release leading to endothelial dysfunction [9]. In a study by Kervinen H et al., it was concluded that patients with raised hs-CRP were at an increased risk for cardiovascular disease [6].
With the given background, we hope to gain a better understanding about the role of iron and hs-CRP in predicting patient outcomes in MI and hence, the present study was conducted to study the association of deranged iron profile and raised hs-CRP in patients diagnosed with STEMI.
Materials and Methods
This was a cross-sectional hospital-based study of in-patients admitted in the Department of General Medicine and Cardiology at Chigateri General Hospital and Bapuji Hospital-JJM Medical College, Davanagere, Karnataka, India. The study duration was of two months (1st April to 31st May 2020). All the patients were residents of Davanagere district Karnataka, belonging to lower socio-economic strata [10].
The study was commenced after the institutional ethical clearance (Number-87-2020). Informed written consent was taken from the patients if they were stable enough for the same. In cases, where patients were critically ill and unable to read and sign the form, the patient’s attenders were asked to sign on their behalf.
The inclusion criteria were defined as patients with:
1) Newly diagnosed acute STEMI in ages between 18 to 85 years, confirmed by echocardiography.
The exclusion criteria were patients with:
1) Old ischemic heart disease,
2) NSTEMI
3) Malignancies,
4) Chronic Kidney Disease (CKD),
5) Patients with acute febrile illness (deranges Hs-CRP)
About 93 patients were screened over the two months and 48 were excluded (CKD-11, malignancy-2, NSTEMI-18, Acute febrile illness-8, Not given consent-9) and 45 patients were finally selected to participate in the study. Primary variables studied were STEMI and site of infarction as confirmed on echocardiography, hospital stay and patient outcome. Secondary variables were serum iron, hs-CRP, TIBC and ferritin. Serum iron profile including Serum Iron, Serum Ferritin, UIBC, TIBC and TS. Hs-CRP was measured by a method called latex enhanced immunoturbidimetric assay (Normal- 0-0.5 mg/dL). Complete Blood Count (CBC) was done by the haematology analyser which is a laser-based measurement. The iron studies were analysed via a dry chemistry method. This is the method used in Bapuji Hospital JJMMC, Davanagere, Karnataka, India. Information was provided by Bapuji Hospital Central Laboratory. CBC, hs-CRP and iron studies were performed on all the 45 patients. Patients were observed during their hospital stay till they survived or succumbed to illness. Site of MI and duration of hospital stay were also noted. History of diabetes, HTN, smoking and alcohol were taken into consideration as they are possible effective modifiers. Patients were followed-up during hospital stay. Possible predictors to be investigated were serum iron levels, TIBC, TS, ferritin, and hs-CRP.
Statistical Analysis
Statistical analysis was done using IBM SPSS software. Results are presented as Mean±SD values for continuous variables and frequency as number and percentages. Since, the measurements were found to be moderately skewed, non-parametric methods were used for analysis. Mann-Whitney U test was used to compare between survivors and deceased groups. Kruskall-Walli’s ANOVA was used for multiple groups (sites of MI) simultaneous comparisons. Categorical data were analysed by Chi-square test. Diagnostic validity tests were performed to predict the prognosis. A confidence interval of 95% was chosen. Correlation was measured by Spearman’s rank correlation coefficient. A p-value of 0.05 or less was considered for statistical significance.
Results
About 45 patients participated in the study (27 males and 18 females). About nine patients succumbed to MI and 36 survived. There were 15 patients with Anterior wall STEMI, nine patients with Anterolateral STEMI, 15 with Inferior wall STEMI, six with Inferolateral wall STEMI. About nine patients had DM, six patients had HTN and six patients had both DM and HTN [Table/Fig-1].
Descriptive information on study subjects.
| Descriptive information on study subjects | Died | Survived |
|---|
| No. of cases | 45 | 9 | 36 |
| Age (Years) | Mean±SD | 63.4±11.8 | | |
| Range | 43-83 | | |
| | No. | % | | |
| Gender | Male | 27 | 60.0 | 2 | 25 |
| Female | 18 | 40.0 | 7 | 11 |
| Diagnosis | 1. Anterior wall STEMI | 15 | 33.3 | 3 | 12 |
| 2. Anterolateral wall STEMI | 9 | 20 | 2 | 7 |
| 3. Inferior wall STEMI | 15 | 33.3 | 1 | 14 |
| 4. Inferolateral STEMI | 6 | 13.3 | 3 | 3 |
| Total | 45 | 100 | 9 | 36 |
| Diabetes Mellitus (DM) only | 9 | 20.0 | 1 | 8 |
| Hypertension (HTN) only | 6 | 13.3 | 0 | 6 |
| Both DM and HTN | 6 | 13.3 | 2 | 4 |
| Neither DM nor HTN | 24 | 53.3 | 6 | 18 |
| Only smokers | 15 | 33.3 | 4 | 11 |
| Only alcoholics | 19 | 42.2 | 3 | 16 |
| Both smoker and alcoholic | 11 | 24.4 | 2 | 9 |
| Hospital stay (days) | Mean±SD | 12.6±3.1 | | 13.67±2.4 | 12.6±3.3 |
| Range | 7-18 days | | 9-18 days | 7-18 days |
About 18 out of 45 (40%) {12 Male and 6 Female} had Serum Iron values below 40 μg/dL, 9 out of 45 (20%) had Serum Iron values between 40-67 μg/dL, and the rest had Iron values above 67 μg/dL. (Normal values=Males-49-181 μg/dL, Females-37-170 μg/dL). Mean serum iron values were normal for anterior wall STEMI and inferior wall STEMI. However, in anterolateral wall STEMI and inferolateral wall STEMI, mean serum iron values were reduced. A p-value of 0.001 was obtained [Table/Fig-2].
Diagnosis-wise comparison of different parameters.
| Diagnosis | Iron (in μg/dL) | TIBC (in μg/dL) | UIBC (in μg/dL) | TS (in %) | Ferritin (in μg/dL) | Hs-CRP (in mg/L) | Red cell distribution width (in %) |
|---|
| Mean | Mean | Mean | Mean | Mean | Mean | Mean |
|---|
| 1. Anterior wall STEMI | 62.9±25.5 | 341.5±88.2 | 297.8±106.8 | 17.5±5.7 | 102.6±58.4 | 2.99±3.46 | 15.3±2.0 |
| 2. Anterolateral wall STEMI | 19.7±4.3 | 228.1±26.8 | 208.0±23.0 | 8.5±1.2 | 269.4±163.9 | 7.47±2.72 | 17.4±4.8 |
| 3. Inferior wall STEMI | 72.1±29.0 | 266.4±36.1 | 250.6±60.4 | 22.5±10.9 | 137.6±79.2 | 2.88±2.11 | 16.6±3.3 |
| 4. Inferolateral STEMI | 40.3±27.8 | 292.3±84.8 | 255.5±60.8 | 12.2±5.8 | 76.5±45.5 | 11.14±11.78 | 15.5±4.8 |
| Overall | 54.3±31.1 | 287.2±75.5 | 258.5±79.9 | 16.7±9.0 | 144.2±112.4 | 4.94±5.62 | 16.2±3.5 |
| Kruskall-Walli’s ANOVA | χ2 | 16.89 | 10.14 | 5.28 | 13.86 | 13.53 | 9.00 | 2.46 |
| p | 0.001* | 0.02* | 0.15, ns | 0.003* | 0.004* | 0.03* | 0.48, ns |
TIBC: Total iron-binding capacity; UIBC: Unbound iron binding capacity; TS: Transferrin saturation; hs-CRP: High sensitive C-reactive protein; STEMI-ST: Segment elevation myocardial infarction; ns- non significant; p<0.05 – significant
It was seen that Serum Iron values lower than 61 mg/dL (cut-off chosen since it was the median value across all study subjects. Due to the high range between highest and lowest values, it was statistically more apt to select the numerical median as the cut-off) had a sensitivity of 89% and specificity of 56% with high NPV of 95% for prediction of mortality in patients [Table/Fig-3].
Diagnostic value of Iron in the prognosis of MI (cut-off value of Iron=61.0).
| Iron | Outcome | Total |
|---|
| Died | Survived |
|---|
| <61.0 mg/dL | 8 (90.0) | 16 (44.4) | 24 (53.3) |
| >61.0 mg/dL | 1 (10.0) | 20 (55.6) | 21 (46.7) |
| Total | 9 (100) | 36 (100) | 45 (100) |
χ2=5.71, p=0.024; p<0.05 - significant
Patients with low serum iron showed prolonged hospital stay (r=-0.60, p=0.01) [Table/Fig-4]. About 27 out of 45 had TIBC lower than 260 μg/dL. Other 18 had normal values (Normal values=Males-261-462 μg/dL, Females-260-497 μg/dL). Anterolateral wall STEMI cases showed low mean TIBC with a significant p-value of 0.02 [Table/Fig-2]. Patients with lower TIBC had prolonged hospital stay (r=-0.41, p=0.01) [Table/Fig-4].
Correlation between serum iron profile and hs-CRP and duration of hospital stay.
| Parameter | Spearman’s R coefficient | p-value | Significance |
|---|
| Hs-CRP | 0.51 | 0.0001 | Significant |
| UIBC | -0.19 | 0.22 | Not significant |
| TS | -0.64 | 0.0001 | Significant |
| Iron | -0.60 | 0.01 | Significant |
| Ferritin | 0.38 | 0.01 | Significant |
| TIBC | -0.41 | 0.01 | Significant |
Spearman Rank r correlation. p<0.05 - significant
TIBC: Total iron-binding capacity; UIBC: Unbound iron binding capacity; TS: Transferrin saturation; hs-CRP: High sensitive C-reactive protein
About 18 out of 45 patients had low TS below 14%. Other patients had normal TS (Normal values=15-50%) Mean TS was low in inferolateral and anterolateral STEMI with a significant p-value of 0.003 [Table/Fig-2]. Patients with low TS had prolonged hospital stay (r=-0.64, p=0.0001) [Table/Fig-4]. About 15 out of 45 had serum ferritin values below 110 μg/dL, 24 had serum ferritin values between 111-219 μg/dL, six had serum ferritin values more than 220 μg/dL (Normal=Males-22-274, Females-10-204). Mean Ferritin was high only in anterolateral wall STEMI with significant p-value of 0.004 [Table/Fig-2]. Higher ferritin values weakly correlated to longer hospital stay (r=0.38, p=0.01) [Table/Fig-4]. The most striking finding was seen with hs-CRP. About 42 out of 45 patients showed hs-CRP levels above 0.3 mg/L. (Normal values- <0.1 mg/L, Average cardiac risk- 0.1-0.3, High cardiac risk- >0.3 mg/L). HS-CRP was found to be high in all types of MI with a p-value of 0.03 [Table/Fig-2].
Hs-CRP was seen to have a sensitivity of 100%, a specificity of 42% and NPV of 100% in predicting mortality (cut-off chosen since it was the median value across all study subjects. Due to the high range between highest and lowest values, it was statistically more apt to select the numerical median as the cut-off) [Table/Fig-5]. Only 6 out of 45 patients had abnormal UIBC values. But it was seen that significantly lower UIBC values were seen in patients who died than the survivor group (p=0.04) [Table/Fig-6]. UIBC showed no significant correlation with the duration of hospital stay [Table/Fig-4].
Diagnostic value of hs-CRP in the prognosis of MI (Cut-off the value of Hs-CRP=2.0).
| Hs-CRP (in mg/L) | Outcome | Total |
|---|
| Died | Survived |
|---|
| >2.0 | 9 (100.0) | 21 (58.3) | 30 (66.7) |
| <2.0 | 0 (0.0) | 15 (41.7) | 15 (33.3) |
| Total | 9 (100) | 36 (100) | 45 (100) |
χ2=5.63; p=0.02; p<0.05 – significant
Initial levels (at admission) of Iron, Ferritin, Hs-CRP, TIBC, UIBC, TS, RDW and other variables relating to outcome.
| Died (9) | Survived (36) | p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Iron (in μg/dL) | 40.0±28.8 | 57.9±31.1 | 0.07, ns |
| TIBC (in μg/dL) | 240.4±19.7 | 298.9±79.9.0 | 0.11, ns |
| UIBC (in μg/dL) | 213.3±36.9 | 269.8±84.0 | 0.04*, S |
| TS (in %) | 15.3±9.4 | 17.0±9.0 | 0.50, ns |
| Ferritin (in μg/dL) | 214.2±157.3 | 126.6±93.0 | 0.15, ns |
| Hs-CRP (in mg/L) | 10.9±8.92 | 3.46±3.19 | 0.008*, S |
| RDW (in %) | 15.9±3.1 | 16.2±3.6 | 0.83, ns |
Mann-Whitney U test is done. p<0.05 - significant
TIBC: Total iron-binding capacity; UIBC: Unbound iron binding capacity; TS: Transferrin saturation; hs-CRP: High sensitive C-reactive protein; RDW: Red cell distribution width
Raised hs-CRP was seen in the deceased as compared to the survivor group [Table/Fig-6]. Raised hs-CRP was also associated with prolonged hospital stay (r=0.51, p=0.0001) [Table/Fig-4].
The remarkable finding of the study is that none of the patients enrolled in the study was anaemic. The investigations were sent after the onset of the MI and no fall in Haemoglobin was seen.
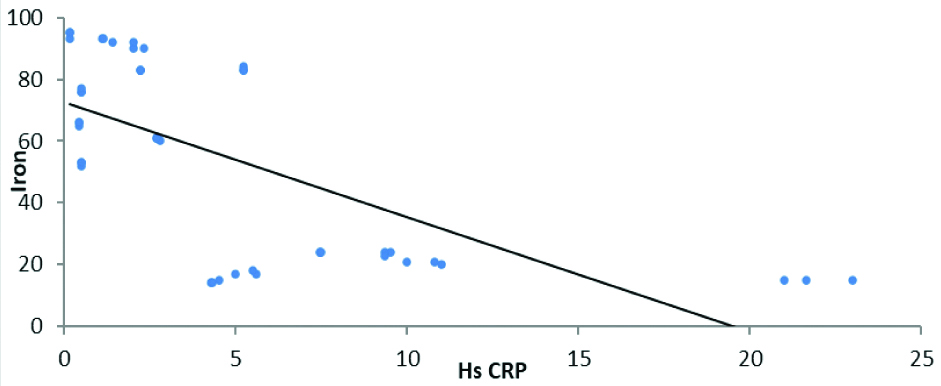
It was seen that lower values of iron were strongly associated with higher values of Hs-CRP [Table/Fig-7] (r=-0.68, p=0.0001).
Depicting relationship between iron values and hs-CRP (r=-0.68, p=0.0001).
Discussion
The protective action of iron has not been completely understood. The well-known role of iron in free-radical-mediated injury is decreased in ischemic myocardial events. Iron depletion protects myocardium by directly inhibiting atherogenesis. Iron depletion can defend against ischemic events, even in patients with considerable atherosclerotic disease [11]. The novel findings put forward that iron could have a clinically more vital function in ischemic events than in initiating or promoting vascular structural lesions. Increased events may occur without an increase in atherosclerotic lesions [12]. Iron deficiency is a common co-morbidity associated with poor prognosis in cardiac conditions. In a study, by Duarte T et al., patients with serum iron values of less than 40 μg/dL had a higher incidence of adverse cardiovascular events [12]. About 18 out of 45 patients had serum iron values less than 40 μg/dL in this study, out of which five patients succumbed to illness with an average hospital stay of 15.1 days as compared to the general average of 12.6 days. This study correlates with the study done by Griffith JD et al., in which it is suggestive that the presence of MI alters the behaviour of plasma iron and is reduced at the time of admission [13]. This study also suggests that the iron remains reduced up till day seven of admission. In this study, however, due to financial constraints of the patient, we could only perform iron studies at admission. Blockage of haemoglobin from the reticuloendothelial system occurs in acute MI with a longer half-life of seven days. Another probable reason could be lactoferrin release leading to leukocyte agranulocytosis. Reduction in TIBC is directly proportional to a reduction in plasma transferrin. A shift of plasma iron into the hepatocyte reduces hepatic transferrin synthesis. The acute changes of iron, transferrin and ferritin lead to a temporary arrest in erythropoiesis leading to a fall in haemoglobin. However, none of the patients had a fall in haemoglobin [13].
TIBC is an independent important negative risk factor for MI. This was derived in a study by Magnusson MK et al., [14]. In the present study, 27 out of 45 patients (60% of patients) had TIBC lower than 260 μg/dL, out of which 9 patients died. Anterolateral wall STEMI cases showed lower mean TIBC with a significant p-value of 0.02 [Table/Fig-2]. Atherosclerotic plaques formation is influenced by the integrity of the fibrous cap due to inflammatory mechanisms. Hs-CRP is an attractive biomarker and has opsonising properties. It pushes the monocytes into the atheroma, suppresses the basal and induced nitric oxide release leading to endothelial dysfunction [9]. Hs-CRP also is an important predictor of type 2 DM. The Indian population is more prone to coronary artery diseases, DM and metabolic syndrome, so, hs-CRP values are likely higher in our country [9]. The striking feature of this study was that mean Hs-CRP was found to be high in all types of MI with a p-value of 0.03. About 42 out of 45 patients showed Hs-CRP levels above 0.3 mg/L. (Normal values- <0.1 mg/L, Average cardiac risk- 0.1-0.3, High cardiac risk- >0.3 mg/L). This study also shows a positive correlation between hs-CRP values with the duration of hospital stay and increased incidence of death. This agrees with a study done by Soinio M et al., which showed that in patients with DM, high hs-CRP was an independent risk factor to predict mortality from coronary heart disease [15]. Oxidative free radicals have a major role in CAD. There is increased peroxidation of LDL, leading to increased uptake by macrophages thereby leading to increased foam cell formation and atherosclerosis. Iron is an especially important constituent and free iron produces free radicals; therefore, it is involved in lipid peroxidation and atherosclerosis leading to Acute MI [15]. In a study by Badiger RH et al., it was observed that serum ferritin levels were higher in patients with acute MI [8]. Mean ferritin was high only in anterolateral wall STEMI in present study. Iron induced lipid peroxidation has been considered as the age-old mechanism. Since inflammation is implied in MI, we expected a significant rise in serum ferritin as it an acute phase reactant. A cohort study with long term follow-up and repeat iron profile and serial hs-CRP monitoring can help establish their role in heart disease.
Limitation(s)
Limitation of the study would be small sample size, lack of follow-up until the next cardiac event and due to financial constraints, no repeat investigations could be performed on patients.
Conclusion(s)
Physicians must be aware of the implications of iron metabolism in the pathogenesis of acute STEMI. Regular iron supplementation with a six monthly hs-CRP monitoring is recommended and can be counted as crucial prognostic tools in acute MI. In patients admitted with acute MI, iron profile and hs-CRP are shown to be important predictors of morbidity and mortality. Physicians must note that these fairly basic and inexpensive measures can go a long way in improving patient care and contribute to preventing morbidity and mortality.
TIBC: Total iron-binding capacity; UIBC: Unbound iron binding capacity; TS: Transferrin saturation; hs-CRP: High sensitive C-reactive protein; STEMI-ST: Segment elevation myocardial infarction; ns- non significant; p<0.05 – significant
χ2=5.71, p=0.024; p<0.05 - significant
Spearman Rank r correlation. p<0.05 - significant
TIBC: Total iron-binding capacity; UIBC: Unbound iron binding capacity; TS: Transferrin saturation; hs-CRP: High sensitive C-reactive protein
χ2=5.63; p=0.02; p<0.05 – significant
Mann-Whitney U test is done. p<0.05 - significant
TIBC: Total iron-binding capacity; UIBC: Unbound iron binding capacity; TS: Transferrin saturation; hs-CRP: High sensitive C-reactive protein; RDW: Red cell distribution width