Worldwide, breast cancer is the commonest invasive malignancy in women representing 24.2% of all invasive cancers in women and account for 15.0% mortality of all female cancers [1,2]. Breast cancer is also the commonest cancer among women in India, surpassing cancer of uterine cervix. In 2018, the incidence rate for breast cancer was 27.7% of all newly detected cancers and accounted for about 23.5% of all cancer related deaths of women in India [3].
Breast cancer is a heterogeneous disease in which therapeutic response and clinical outcome varies despite similarities in histologic types, grade and stage. Reason for these differences are not well known but may be due to the limitations in our current taxonomy of breast cancer, which classifies molecular distinct groups into clinical class based on morphology. A partial explanation for this disparity is found in studies using modern techniques including molecular profiling to examine the biological underpinning of breast cancer [4-6]. The biological evidence of heterogeneity is provided by molecular profiling. Global Gene Expression Profiling (GEP) by Perou CM et al., and Sorlie T et al., led to molecular classification of breast carcinomas which categorised breast carcinomas into five intrinsic subtypes (Luminal A, Luminal B, Basal like, HER2 Overexpression, normal breast like) with different clinical outcomes and response to neo-adjuvant chemotherapy [7,8]. Since then several additional subtypes have been proposed.
Since GEP is neither economical nor practical in routine practice, many investigators have used IHC based molecular classification to study the invasive breast cancer and have shown predictive and prognostic value comparable with that of GEP. Breast carcinomas can be divided into five similar subgroups using IHC analysis as a surrogate with limited panel antibody markers (including ER, PR, HER2, CK5/6 and EGFR). These are hormone positive (ER and/or PR positive) Luminal group and hormone negative non-luminal group. The ‘luminal class’ further sub-divided into Luminal A and Luminal B, depending on presence or absence of HER2 positivity.
Luminal A class has favourable clinical features among the five subtypes while Triple Negative Breast Carcinoma (TNBC)/Basal Like Breast Carcinomas (BLBC) have poor prognosis and therapy response. TNBCs are ER PR and HER negative tumours thus include both BLBC and normal breast like subtypes. Although the histologic and IHC phenotypes of TNBCs and BLBC’s overlap, the terms “triple-negative” and “basal-like” are not synonymous as often perceived. A more complete definition of BLBC includes expression of basal cytokeratins (CK5/6, 14, 17) and/or over-expression of human epidermal growth factor receptor 1 (HER-1/EGFR) [9]. Recent evidence suggests that TNBC represent a more heterogeneous group than BLBC [10]. The aggressive behaviour of these TNBC is due to the co-expression of basal markers [11]. Basal subtypes are more prevalent in patients with BRCA1 mutation [12]. BLBC and HER2 subtypes are associated with poor clinical outcomes [8,12].
Present study was undertaken to identify and document the prevalence of molecular subtypes of invasive breast carcinoma using IHC as described in literature and to study the morphological features of each subtype, to identify the poor prognostic subtype by light microscopy on routine histological examination. The morphological features and prognostic parameters i.e., tumour size, tumour type, tumour grade, Lymphovascular Emboli (LVE) and lymph node status of invasive breast carcinoma of each subtype were studied.
Materials and Methods
The present prospective cross-sectional observational study was based on mastectomy specimens of 122 cases of breast carcinoma, who did not receive any chemotherapy or underwent lumpectomy submitted in Department of Pathology, Institute of Medical Sciences, Banaras Hindu University and diagnosed as Breast carcinoma on histopathology from June 2012 to February 2014. The Institute Ethics Committee of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, approved the study (ECR/526/Inst/UP/2014 dt 30.01.2014). The bio-clinical data like age, laterality, menopausal status, parity, and other relevant parameters were obtained from case records. Mastectomy specimens were processed for formalin fixed paraffin embedded sections for routine Haematoxylin and Eosin staining and immunohistochemistry. The sections were examined under light microscope for various histopathological features including histopathological type, tumour grade, tubule formation, nuclear pleomorphism, mitotic index, lymphovascular invasion, necrosis, carcinoma in situ, lymphocytic infiltration. Other features like foreign body giant cell reaction, areas of hyalinisation and sclerosis, features of fibrocystic disease, amyloid deposit and columnar cell metaplasia were noted. Representative sections from all the cases were selected and were subjected for IHC for ER, PR, HER2 neu, CK5/6 and EGFR using the antibodies listed in [Table/Fig-1]. Adjacent normal breast parenchyma was used as internal positive control. Negative controls were obtained by omitting the primary antibodies.
Details of antibodies used in the study.
| Antibody | Clone | Dilution | Company |
|---|
| ER | SP1 | Ready to use | Thermo Fisher Scientific |
| PR | SP2 | Ready to use | Thermo Fisher Scientific |
| HER2 neu | SP3 | Ready to use | Thermo Fisher Scientific |
| CK5/6 | EPR1600Y and EPR1602Y | Ready to use | BioGenex |
| EGFR | EP38Y | Ready to use | BioGenex |
ER/PR immunoreactivity was interpreted quantitatively as presence or absence of nuclear reactivity of the tumour cells, percentage of positive stained nuclei and intensity of staining was recorded separately according to Allred score. A total score of equal to or more than 4 were interpreted as ER/PR positive tumours and score equal to or less than 4 were interpreted as ER/PR negative tumours. Results of HER2/neu expression were interpreted according to College of American Pathologists (CAP) guidelines [13,14]. A score of 3+ was considered as positive whereas the cases with 1+ or 2+ were considered negative. EGFR- cytoplsamic expression of any intensity in more than 1% of cells was considered as positive basal marker [15]. Epidermal layer of skin considered as positive control. For CK5/6 any cytoplasmic expression in definite neoplastic cells or tissue was considered as positive result [15].
Results of IHC in each case were compiled and the cases were categorised into five molecular subtypes according to immunophenotype. Luminal A Luminal B, HER2 overexpression, BLBC and unclassified (normal breast like subtypes) [7,8].
Statistical Analysis
All statistical analysis were performed using SPSS version 16 (SPSS, Inc., Chicago, IL, USA). Analysis Chi-square Pearson was used to find the association between various histopathological variables with various molecular subtypes. Fisher’s-Exact test was used when expected cell counts were less than 5. A p-value <0.05 was considered as statistically significant. Odds Ratios (ORs) and 95% Confidence Intervals (CIs) were also calculated wherever applicable to estimate the magnitude of association among breast cancer subtypes.
Results
A total of 122 consecutive cases of modified radical mastectomy specimens were received from June 2012 to February 2014 that were diagnosed as Invasive breast carcinoma were included in the study. The age of the patients ranged between 20 to 70 years with mean age of 47.5±9.89 years and median age was 48 years. Peak incidence (48; 39.34%) was observed in the age group 41 to 50 years. On gross examination the maximum diameter of tumour ranged from 1 cm to 15 cm while the mean size was 4.51 cm. Multicentric tumour was observed in 6.56% (8) patients had while rest of the 93.44% (114) had single focus of tumour. Out of 122 cases, 119 were reported as Infiltrating Ductal Carcinoma (IDC), 2 cases as medullary carcinoma and one case as Inflitrating Lobular Carcinoma (ILC). All the 119 cases of IDC were classified according to modified Bloom-Richardson grading system into grade 1, 2 and 3. Majority were categorised to IDC grade 2 (60.50%), 26.89 as grade 3 while rest as grade 1 [Table/Fig-2a,b and c].
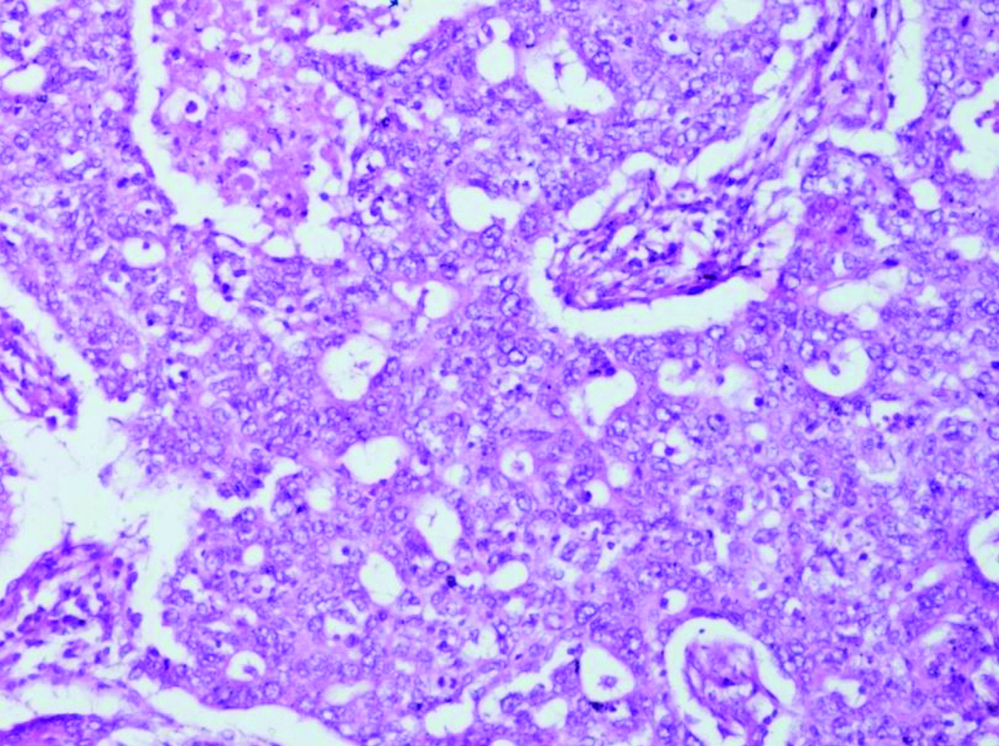
Photomicrograph showing infiltrating ductal carcinoma- grade I showing tubular formation (60-70%) with mild nuclear pleomorphism (H&E Stain X 400).
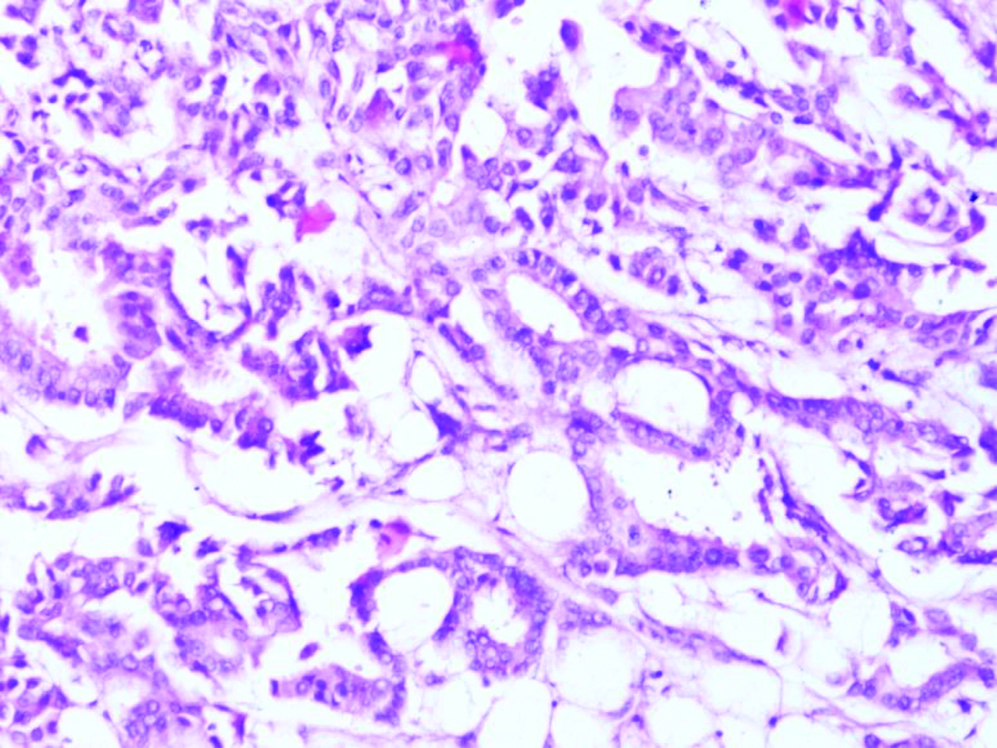
Photomicrograph of infiltrating ductal carcinoma- grade II showing tubular formation (30-40%) with moderate nuclear pleomorphism (H&E Stain X 400).
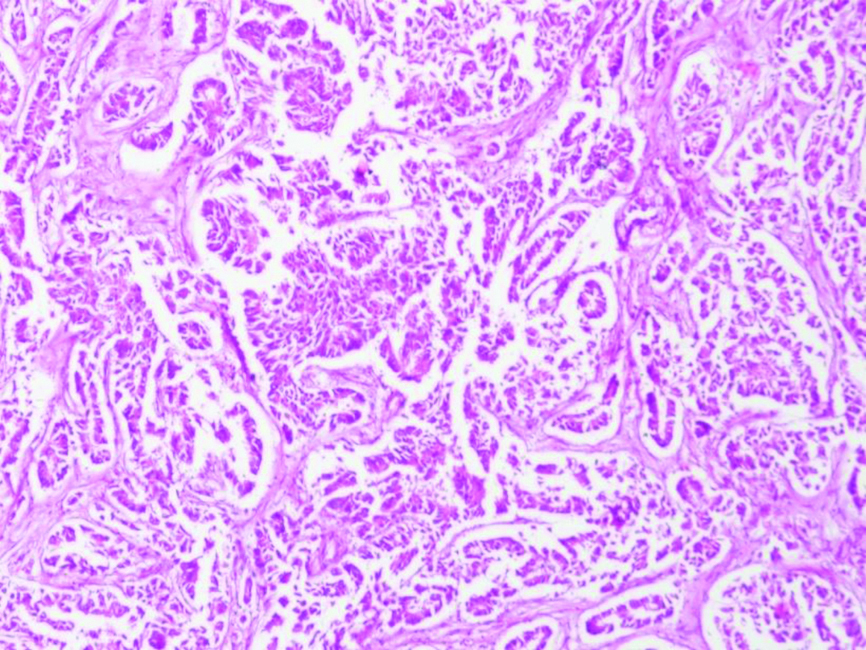
Photomicrograph of a case of infiltrating ductal carcinoma- grade III showing tubular formation (<10%) with marked nuclear pleomorphism high mitosis (H&E Stain X 400).
Results of immunohistochemical examination revealed that ER was positive in 36.89% (45) cases [Table/Fig-2d] and negative in 63.11% (77) cases. PR was positive in 32.79% (40) cases [Table/Fig-2e] while in 67.21% (82) cases it was negative. HER2 was positive in 42.62% (52) cases [Table/Fig-2f] while it was reported negative in 57.38% (70) cases. EGFR was positive in 82.79% (101) cases [Table/Fig-2g,h] while it was negative in 17.21% (21) cases. CK5/6 showed positivity in 57 (46.72%) cases [Table/Fig-2i] while negative in 65 (53.28%) cases. According to predetermined criteria 28.69% (35) of cases were categorised as luminal A subtype (ER/PR + and HER-), 17.21% (21) patients as luminal B (ER/PR+ and HER2+), 25.41% (31) patients as HER2 over expressing, (ER and PR – and HER2 +ve), 26.23% (32) patients as BLBC (ER, PR and HER2 –ve and EGFR/CK5/6 +ve) and the rest 2.46% (3) patients as unclassified category (normal breast like) (ER, PR, HER2, CK5/6 and EGFR all negative).
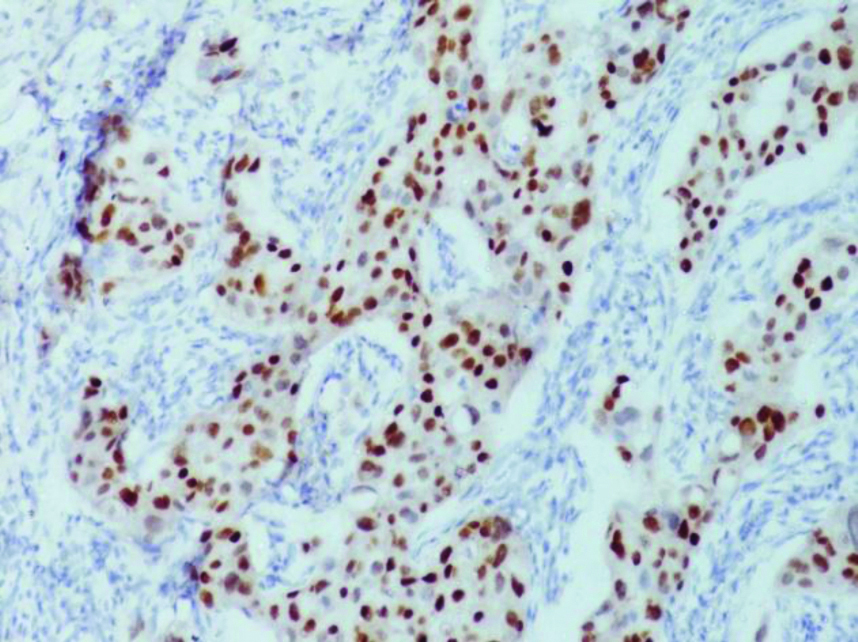
Immunohistochemistry for estrogen receptor showing strong nuclear staining in >50% of the tumour cell nuclei in a case of breast carcinoma (IHC-ER X400).
Immunohistochemistry for progesterone receptor on tissue section showing strong nuclear staining in >50% of the tumour cell nuclei (IHC-ER X400).
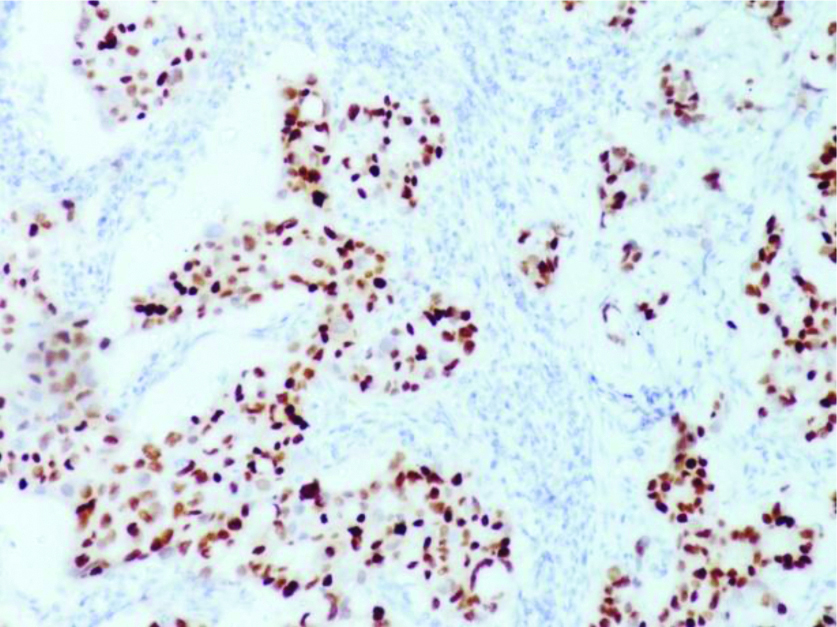
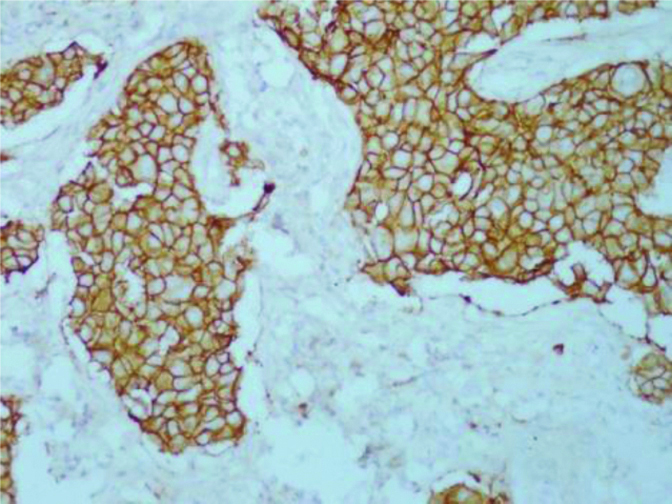
Photomicrograph showing results of immunohistochemistry for HER2. Intense complete membranous. staining in >70% tumour cells interpreted as over-expression (3+) in a case (IHC-HER2 X 400).
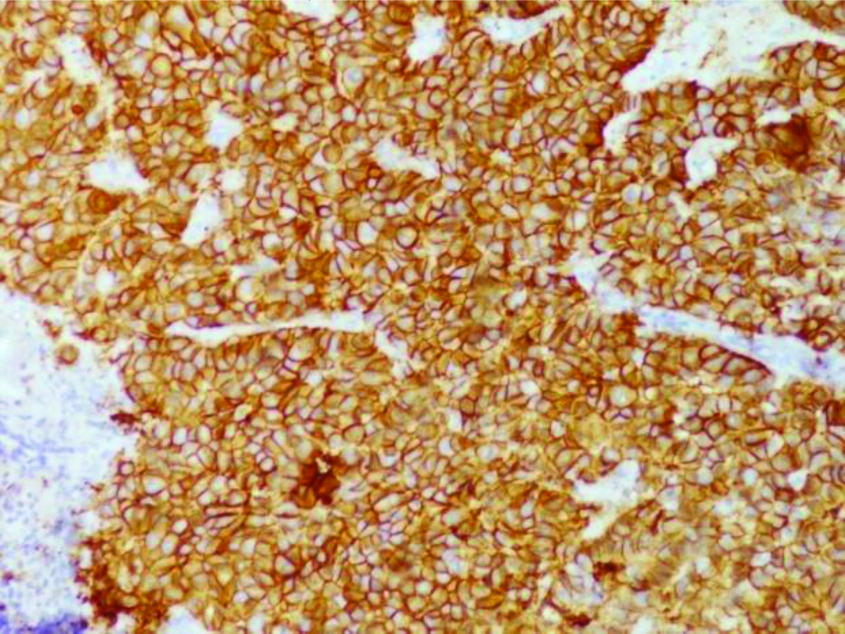
Photomicrograph showing results of immunohistochemistry for EGFR. Cytoplasmic staining with in >50% tumour cells with mild incomplete membranous staining, interpreted as 1+ staining for EGFR (IHC-EGFR X 400).
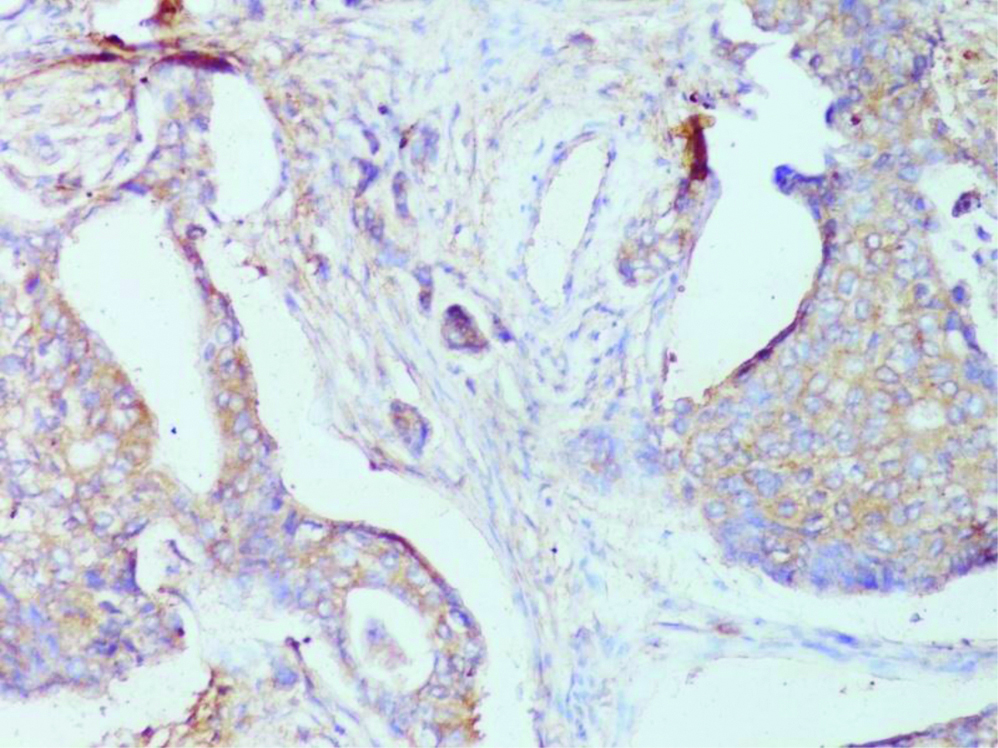
Photomicrograph showing results of immunohistochemistry for EGFR. Moderate and incomplete membrane staining in >50% tumour cells recorded as 2+ expression for EGFR. (IHC-EGFR X 400).
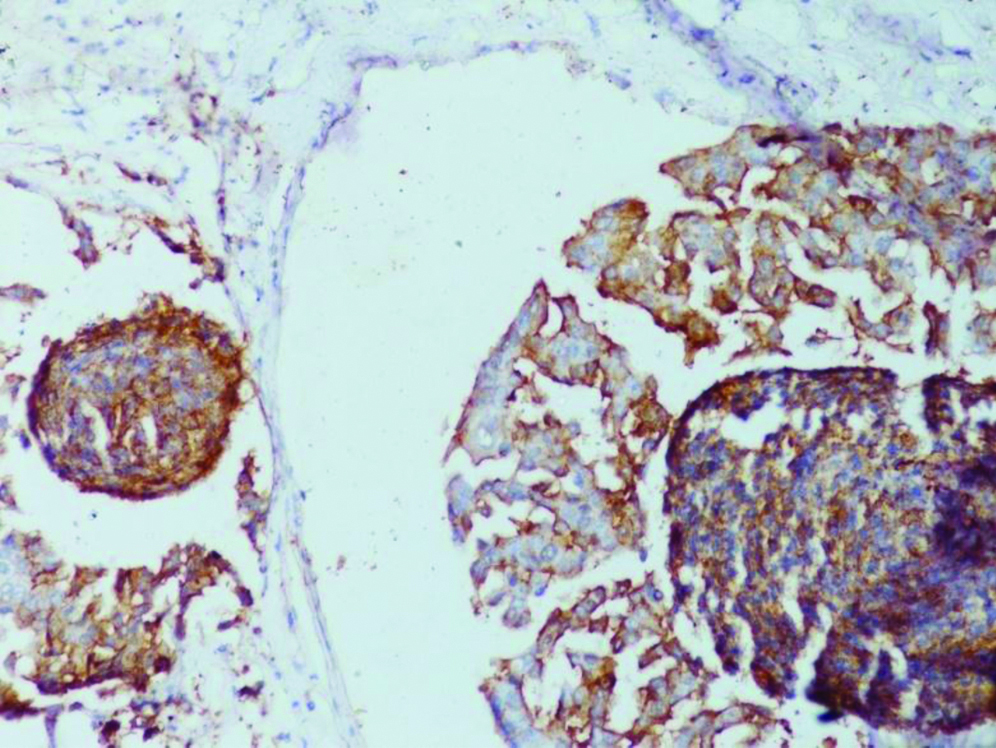
Photomicrograph showing results of immunohistochemistry for CK5/6 expression with cytoplasmic (membranous) positivity staining in >30% tumour cells in tissue section (IHC-CK5/6 X 400).
[Table/Fig-3] summarises the clinicopathological findings of five subclasses. Statistically significant difference was observed between BLBC when were compared with luminal type of carcinoma (Luminal A and Luminal B) which were larger, poorly differentiated, had higher mitotic index, with more geographic and central necrosis and more number of positive lymph nodes than Luminal A group (p<0.05). Larger tumour diameter and multifocal tumours were observed in BLBC and HER2 overexpression class than the luminal class. Poorly differentiated tumours with higher mitotic index, more commonly associated with geographic and central necrosis observed to be more frequent in BLBC class than HER2 over expressing group and luminal group. These findings were statistically significant (p<0.05). No significant difference in the mean and median of age of the two groups was found [Table/Fig-3].
Clinicopathological characteristics of molecular subclass of breast carcinoma.
| Variables | | Luminal A (n=35) | Luminal B (n=21) | HER-2 overexpression (n=31) | BLBC (n=32) | Unclassified (n=3) | All cases (n=122) | p-value |
|---|
| Age | Mean/Range | 47.70/20-65 | 47.6/28-62 | 47.8/30-70 | 47.6/30-70 | 43.3/40-45 | 47.5/20-70 | 0.963 |
| Tumour size | <2 cm/2-5 cm | 3 (8.57%)/22 (62.86%) | 5 (23.81%)/12 (57.14%) | 3 (9.68%)/18 (58.06%) | 1 (3.12%)/15 (46.88%) | 1 (33.33%)/1 (33.33%) | 12 (9.84%)/70 (57.38%) | 0.045 |
| >5 cm | 10 (28.57%) | 4 (19.05%) | 10 (31.25%) | 16 (50.00%) | 1 (33.33%) | 40 (32.79%) | |
| Centrality | Single/Multiple | 33 (94.29%)/2 (5.71%) | 20 (95.24%)/1 (4.76%) | 29 (93.55%)/2 (6.45%) | 31 (96.88%)/1 (3.13%) | 1 (33.33%)/2 (66.67%) | 114 (93.44%)/8 (6.56%) | 0.001 |
| Histological type | Infiltrating Ductal Carcinoma | 35 (100.00%) | 21 (100.00%) | 30 (96.77%) | 30 (93.75%) | 3 (100.00%) | 119 (97.54%) | 0.856 |
| Inflitrating Lobular Carcinoma/Medullary Carcinoma | 0 (0.00%)/0 (0.00%) | 0 (0.00%)/0 (0.00%) | 1 (3.23%)/0 (0.00%) | 1 (3.13%)/1 (3.13%) | 0 (0.00%)/0 (0.00%) | 1 (0.82%)/2 (1.64%) | |
| Nuclear pleomorphism (N=119)** | Mild/Moderate | 3 (8.82%)/25 (73.53%) | 0 (0.00%)/17 (80.95%) | 4 (12.90%)/16 (51.61%) | 3 (9.39%)/13 (40.63%) | 0 (0.00%)/3 (100.00%) | 3 (2.52%)/80 (67.23%) | 0.047 |
| Marked | 7 (20.00%) | 4 (19.05%) | 11 (35.48%) | 16 (50.00%) | 0 (0.00%) | 36 (30.25%) | |
| Mitotic index (N=119)** | <10/10 hpf | 28 (80.00%) | 16 (76.19%) | 10 (31.25%) | 16 (50.00%) | 0 (0.00%) | 78 (65.55%) | 0.047 |
| >10/10 hpf | 7 (20.00%) | 5 (23.81%) | 21 (68.75%) | 16 (50.00%) | 3 (100.00%) | 41 (34.45%) | |
| Lymph node Positive | 0/1-4 | 8 (22.86%)/17 (48.57%) | 4 (19.05%)/12 (57.14%) | 11 (35.48%)/9 (29.03%) | 7 (21.88%)/12 (37.50%) | 1 (33.33%)/1 (33.33%) | 11 (9.02%)/21 (17.21%) | 0.721 |
| >4 | 10 (28.57%) | 5 (23.81%) | 11 (35.48%) | 13 (40.63%) | 1 (33.33%) | 90 (76.92%) | |
| Lympho-Vascular Invasion | Positive/Negative | 24 (68.57%)/11 (31.43%) | 12 (57.14%)/9 (42.86%) | 11 (35.48%)/20 (64.52%) | 16 (50.00%)/16 (50.00%) | 3 (100.00%)/0 (0.00%) | 75 (61.48%/47 (38.52%)) | 0.882 |
| Necrosis | Negative/Central | 8 (22.86%)/26 (74.29%) | 1 (4.76%)/18 (85.71%) | 4 (12.90%)/21 (69.75%) | 4 (12.50%)/16 (50.00%) | 1 (33.33%)/0 (0.00%) | 19 (15.57%)/80 (65.57%) | 0.003 |
| Geographic | 1 (2.86%) | 2 (9.52%) | 6 (19.35%) | 12 (37.50%) | 2 (66.67%) | 23 (18.85%) | |
| Ductal carcinoma in situ | + | 17 (48.57%) | 7 (33.33%) | 11 (35.48%) | 14 (43.75%) | 0 (0.00%) | 49 (40.16%) | 0.337 |
| - | 18 (51.43%) | 14 (66.67%) | 20 (64.52%) | 18 (56.25%) | 3 (100.00%) | 73 (59.84%) | |
| Lymphocytic infiltration | Mild (<25%) | 20 (57.14%) | 13 (61.90%) | 16 (51.61%) | 18 (56.25%) | 2 (66.67%) | 68 (55.74%) | 0.859 |
| Moderate (25-50%)/Marked (>50%) | 15 (42.86%)/0 (0.00%) | 7 (33.33%)/1 (4.77%) | 13 (41.94%)/2 (6.45%) | 13 (40.63%)/1 (3.13%) | 1 (33.33%)/0 (0.00%) | 50 (40.98%)/4 (3.28%) | |
| ER | + | 30 (85.71%) | 15 (71.43%) | 0 (0%) | 0 (0%) | 0 (0%) | 45 (36.89%) | 0.001 |
| - | 5 (14.29%) | 6 (28.57%) | 31 (100%) | 32 (100%) | 3 (100%) | 77 (63.11%) | |
| PR | + | 25 (71.43%) | 15 (71.43%) | 0 (0%) | 0 (0%) | 0 (0%) | 40 (32.79%) | <0.001 |
| - | 10 (28.57%) | 6 (28.53%) | 31 (100%) | 32 (100%) | 3 (100) | 82 (67.21%) | |
| HER2 | + | 0 (0.00%) | 21 (100%) | 31 (100%) | 0 (0%) | 0 (0%0 | 52 (42.62%) | <0.001 |
| - | 35 (100.00%) | 0 (0.00%) | 0 (0%) | 32 (100%) | 3 (100%) | 70 (47.38%) | |
| CK5/6 | + | 12 (34.29%) | 6 (28.57%) | 12 (38.71%) | 27 (84.38%) | 0 (0%) | 57 (46.72%) | 0.001 |
| - | 23 (65.71%) | 15 (71.43%) | 19 (61.29%) | 5 (15.62%) | 3 (100%) | 65 (53.28%) | |
| EGFR | + | 29 (82.86%) | 19 (90.48%) | 24 (77.42%) | 29 (90.63%) | 0 (0%) | 101 (82.79%) | 0.002 |
| - | 6 (7.14%) | 2 (9.52%) | 7 (22.58% | 3 (3.37%) | 3 (100%) | 21 (17.21%) | |
*Fishers exact test LVI-Lymphovascular Invasion **119 cases of IDC taken into consideration, 2 cases of Medullary carcinoma and 1 cases of Lobular carcinoma excluded; p<0.05- statistically significant.
On comparing the cilnicopathological features of ER/PR+ and ER/PR- tumours [Table/Fig-4], it was found that tumour size of ER/PR- group was larger than ER/PR+ group. This category also showed poor differentiation, higher mitotic index, higher number of metastatic lymph nodes than ER/PR+ group and these findings were statistically significant (p<0.05).
Clinicopathological Characteristics of ER/PR positive and ER/PR negative Breast Carcinoma.
| Variables | ER/PR+ (n=56)** | ER/PR- (n=66)*** | p-value |
|---|
| Age (years) | Mean | 47.5 | 47.5 | 0.622 |
| Range | 20-70 | 30-70 | |
| Tumour size | <2 cm | 8 (14.29%) | 5 (7.58%) | 0.134 |
| 2-5 cm | 34 (60.71%) | 34 (51.51%) | |
| >5 cm | 14 (25.00%) | 27 (40.91%) | |
| Centrality | Single | 53 (94.64%) | 61 (92.42%) | 0.622 |
| Multiple | 3 (5.35%) | 5 (7.58%) | |
| Differentiation | Well/moderate | 47 (83.92%) | 40 (60.61%) | 0.012 |
| Poor | 9 (16.07%) | 26 (39.39%) | |
| Nuclear grade | Mild/moderate | 45 (80.35%) | 38 (57.58%) | 0.011 |
| Marked | 11 (19.64%) | 28 (42.42%) | |
| Mitotic index | <10/10 hpf | 43 (76.78%) | 35 (53.03%) | 0.008 |
| >10 hpf | 13 (23.22%) | 31 (46.97%) | |
| Lymph node Positive | 0 | 12 (21.42%) | 19 (28.79%) | 0.023 |
| 1-4 | 29 (51.78%) | 22 (33.33%) | |
| >4 | 15 (26.78%) | 25 (37.88%) | |
| LVI | + | 36 (64.29%) | 39 (59.09%) | 0.345 |
| - | 20 (35.71%) | 27 (40.91%) | |
| Necrosis | Negative | 9 (16.07%) | 9 (13.64%) | 0.002 |
| Central | 44 (78.57%) | 37 (56.06%) | |
| Geographic | 3 (5.36%) | 20 (30.30%) | |
| Ductal carcinoma in situ | + | 24 (42.86%) | 25 (37.88%) | 0.354* |
| - | 32 (57.14%) | 41 (62.12%) | |
| Lymphocytic Infiltration | Mild | 33 (59.92%) | 26 (39.39%) | 0.661 |
| Moderate | 22 (39.39%) | 37 (56.07%) | |
| Marked | 1 (1.79%) | 3 (4.54%) | |
| HER2 neu | + | 20 (35.71%) | 31 (46.97%) | 0.142* |
| - | 36 (64.39%) | 35 (53.03%) | |
*Fishers-exact test **Either ER or PR or Both Positive ***Both ER and PR Negative; p<0.05- statistically significant
Statistical analysis was carried out to evaluate the association of the histopathological features among the tumour subtypes. HER2 overexpressing, BLBC, unclassified and Luminal B tumour subtypes were compared with Luminal A and OR was calculated with 95% CI. HER2 over expressing group were times more likely to have nuclear grade of poorly differentiated carcinoma (OR 3.75, p=0.037) and higher mitotic index (OR 2.571, p=0.09) than luminal class. BLBC subtypes were more likely to be poorly differentiation (OR 4.667; p=0.006), show marked anisonucleosis (OR 7.5; p=0.001) and more likely to have higher mitotic index (OR 4.408; p=0.006) than Luminal A group and these findings were statistically significant [Table/Fig-5]. No significant difference in the menopausal status, differentiation of tumour, anisonucleosis, mitotic index and lymphovascular infiltration between Luminal A and Luminal B group.
Association of tumour characteristics and molecular class. *Fisher exact test; p<0.05- statistically significant
| Characteristics | HER2 | p-value | BLBC | p-value | Luminal B | p-value | Unclassified | p-value |
|---|
| Odds ratio | Odds ratio | Odds ratio | Odds ratio |
|---|
| Menopausal (post vs pre) | 1.500 | 0.317* | 0.996 | 0.598* | 0.848 | 0.497* | 1.043 | 0.735* |
| Histopathological differentiation grade (poor vs. well/moderate) | 1.420 | 0.399* | 4.667 | 0.006 | 0.778 | 0.527* | 1.107 | 0.427 |
| Nuclear grade (poor vs. mild/moderate) | 3.750 | 0.037 | 7.500 | 0.001 | 2.344 | 0.241 | 1.100 | 0.529 |
| Mitotic index >10/10 hpf vs. <10/10 hpf | 2.571 | 0.090 | 4.408 | 0.006 | 1.205 | 0.779 | 1.111 | 0.383 |
| LVI (+ vs. -) | 1.200 | 0.465* | 2.182 | 0.097* | 1.636 | 0.388 | 1.091 | 0.692* |
Discussion
Invasive breast carcinomas are heterogeneous disease that has distinct molecular and pathological features and biological behaviour. Prognosis and clinical outcomes of the patient is predicted by certain clinical parameters as well as histopathological variables like tumour size, tumour grade, lymph node status and routinely hormone receptors status ER, PR, HER2 neu [16-19]. However, these indicators are not adequate enough as there are discrepancies in the patient outcome within similar prognostic category. Therefore there was need to explore accurate prognostic indicators which were identified by molecular profiling of breast cancers to great extent [4,8,13,20-23].
IHC analysis of ER, PR, and HER2 are critical for IHC based classification [24] and immunoreactivity for cytokeratin 5/6(CK 5/6) and EFGR help to identify the BLBC among the TNBC [12,19,25]. The term BLBC and TNBC have been used often interchangeably, however, they are not synonymous. Two distinct type of epithelial cells are found in Human mammary glands: basal (and/myoepithelial) cells and luminal epithelial cells. CK5/6 is a high molecular weight cytokeratin and is expressed in normal myoepithelial cells. Nielsen TO et al., documented that a panel of four markers (ER, PR, HER2 and CK5/6) accurately identifies BLBC [25]. CK5/6 is often expressed in BRCA1 related breast cancers [20]. EGFR however has enhanced the identification of BLBC because of higher expression and easy scoring than CK5/6 [25]. Cheang MC et al., have used a 5-biomarker immunohistochemistry panel containing oestrogen receptor, progesterone receptor, HER2/neu, CK5/6 and EGFR to demonstrate superior prognostic value than solely the triple-negative phenotype [12]. In the present study, five IHC surrogate markers ER, PR, HER2, CK5/6 and EGFR were evaluated on 122 cases of breast cancers to identify the molecular subtypes.
The results of various molecular subtypes by different authors have been summarised in [Table/Fig-6] [21-34]. Luminal A emerges as largest single subtype in most of the series however the proportion varies 27% to 73%. In present study, 28.69% patients were categorised as Luminal A, which was almost similar to the results reported by Tiwari S et al., (27.1%) and Huo D et al., (27%) [23,26]. BLBC subtype (26.23%) emerged as second largest in the present study. BLBC comprises 7.4% to 32% of all breast cancers in the studies reported in literature. Present study findings were similar to the results of Huo D et al., [26]. The proportion of patients in HER2 group varied from 7% to 29% among various series. In the present study on North Indian patients we found 25.41% patients which was similar to findings of Tiwari S et al., Nielsen TO et al., and Gupta S et al., which had 25.7% 23%, and 24% patients respectively in this group [23,25,27]. Luminal B group in present study included 17.21% patients which was similar to the findings of Carey LA et al., and Fan C et al., which had 16% and 19% patients, respectively [21,28]. Other study on Indian population, Tiwari S et al., Gupta S et al., and Munjal K et al., who reported 35.7%, 21.3% and 11% patients in this group [23,27,29]. The unclassified group had only three patients in the present study and this proportion was less as compared to other studies except Carey LA et al., who did not find any patient to this group [21]. Nielsen TO et al., reported highest 22% patients to this group [25].
Comparative findings of different studies on Breast Cancer molecular subtypes in various studies [21-34].
| Study | Total | Luminal A | Luminal B | Luminal (A+B) | HER2 overexpression | Basal like | Unclassified |
|---|
| Nielsen TO et al., [25] | 663 | - | - | 40% | 23% | 15% | 22% |
| Carey LA et al., [24] | 496 | 51% | 16% | 67% | 7% | 20% | 6.2% |
| Livasy CA et al., [22] | 245 | 61% | 23% | 84% | 16% | 8% | 6% |
| Fan C et al., [28] | 295 | 42% | 19% | 61% | 12% | 18% | 9.8% |
| Carey LA et al., [21] | 107 | 33.64% | 24.30% | 57.94% | 10.28% | 31.78% | 0% |
| Yang XR et al., [31] | 804 | 69% | 6% | 75% | 8% | 12% | 6% |
| Huo D et al., [26] | 507 | 27% | 2% | 29% | 15% | 27% | 28% |
| Spitale A et al., [32] | 1214 | 73% | 13% | 86% | 5.6% | 7.4% | -- |
| Salhia B et al., [33] | 359 | 44% | 26.6% | 70.6% | 11.8% | 11.3% | 7.9% |
| Munjal K et al., [29] | 107 | 37% | 11% | 48% | 29% | 7.5% | 15% |
| Munirah MA et al., [30] | 217 | 57.6% | 6.9% | 64.5% | 14.3% | 7.4% | 13.8% |
| Gupta S et al., [27] | 75 | 45.3% | 21.3% | 66.6% | 24% | 0% | 9.3% |
| Tesfamariam et al., [34] | 80 | 55% | 5% | 60% | 5% | 10% | 25% |
| Tiwari S et al., [23] | 70 | 27.1% | 25.7% | 52.8% | 25.7% | 15.7% | 5.7% |
| Present study | 122 | 28.69% | 17.21% | 45.90% | 25.41% | 26.23% | 2.46% |
Determination of hormonal status is an important primary assessment in evaluation of histopathology samples at the time of a breast cancer diagnosis. These biomarkers have been routinely used in clinical practice to assess patients with breast cancer for predicting prognosis and planning treatment. Present study categorised 56 tumours ER/PR positive and 66 as ER/PR negative (54.10%). On comparing ER/PR negative tumours were found to be associated with poor differentiation (p<0.001), high nuclear grade (p=0.01) high mitotic index (p=0.008) and higher positive lymphnodes (p=0.023) when compared with the ER/PR positive tumours. Expression of ER or PR generally is associated with a better outcome. Survival and response to hormone therapy are most favourable among women with tumours positive for both ER and PR, least favourable for tumours negative for both [34-36].
On comparing the various clinicopathological parameters across the molecular subtypes we observed that the patients in BLBC group and HER2+overexpression group presented with larger tumour size than the patients of luminal group (p=0.045). Similar observations were also reported in the study of Gupta S et al., (<0.001), Munirah MA et al., (p=0.009) [27,30], however it was statistically insignificant (p=0.140) in the study of Munjal K et al., [29]. The tumours of BLBC group were poorly differentiated (p=0.047), had higher mitotic index (p=0.047), than Luminal A group. Munjal K et al., and Munirah MA et al., documented higher number of metastatic lymph nodes in the BLBC and HER2+ overexpression subtypes [(p=<0.050 Munjal K et al.,) [29]; (p=0.032 Munirah MA et al.,) [30], whereas though higher number of positive lymph nodes were observed in present study, these results were stastically not significant. Further authors observed that tumours in BLBC and HER2 overexpressive group displayed features of high grade tumours as compared luminal class (p=0.002). Similar relationship were also observed in studies by Gupta S et al., (p<0.001), Munjal K et al., (p<0.001), and Munirah MA et al., (p=0.000) [27,29,30].
Analysing the association of clinicopathological variable among the various subclass it was observed that HER2 over expressing group were 3.75 times more likely to have poorly differentiated carcinoma and 2.571 times more likely to have higher mitotic index than luminal class (p<0.05). Also, BLBC subtypes were 4.667 times more likely to be poorly differentiated, 7.5 times more likely to have marked anisonucleosis and 4.408 times higher mitotic index than luminal A group (p=0.006). Thus this can be concluded that these microscopic features are strongly associated with BLBC subtype and HER2 overexpression subtypes.
Association of morphological features such as tumour size high histological grade and marked mitotic activity with BLBC phenotype was observed by Verma S et al., [37]. Further they commented that these morphological features did not differ in Triple negative and HER2/neu group in their study. Literature review has shown that BLBC are poorly differentiated invasive ductal carcinomas with nuclear grade III, brisk mitoses, and no tubule formation. These tumours often show a solid growth pattern with pushing borders, areas of geographic necrosis, and a dense lymphocytic stromal infiltrate [39].
BLBCs are aggressive tumours with poor prognosis when compared with other breast carcinomas. Several studies have shown a decreased disease free survival, disease-specific survival, and overall survival and increased risk for early relapses or recurrence in BLBCs compared to those associated with other intrinsic molecular subtypes of breast cancer [38]. Multiple studies have identified basal CKs expression as a poor prognostic factor independent of tumour size and grade in node-negative breast carcinomas [39]. High expression of basal CKs has been considered as the Sine qua non of BLBC. CK5/6 expression has been observed in 73% of BLBC, followed by CK14 (44%) and CK17 (33%) whereas expression of EGFR in BLBC varies from 45% to 70% in several studies [18,38,40]. We observed CK 5/6 expression in 84.38% of tumours and EGFR in 90.63% of BLBC. CK5/6 and EGFR, along with ER and HER2/neu, has been shown to identify BLBC with 100% specificity and 76% sensitivity [25]. Molecular studies (GEP) studies [40] have shown that more than 70% of BRCA1-associated cancers cluster in the BLBC category. Altered and loss of function of BRCA1 activity have been shown in many sporadic BLBCs [41]. Approximately, 10% to 20% of BLBCs show promoter methylation of the BRCA 1 gene, and a subset shows decreased BRCA1 messenger RNA (mRNA) levels [42]. Furthermore, BLBC continues to pose the greatest treatment challenge as compared to other molecular subtypes because of its aggressive clinical course and a lack of targeted therapy.
Limitation(s)
The limitation of present study was that while evaluating HER2 expression cases with 2+ (equivocal) expressions were considered as negative and were not evaluated further with molecular test like Fluorescent In Situ Hybridisation (FISH) as done in some other studies. Therefore, probably some of the HER2 over expressing cases might have been missed and resulting into categorisation of these cases as BLBC or Luminal A type.
Conclusion(s)
Identification of molecular subtype of breast cancer is extremely important for predicting prognosis and therapeutic response of the breast cancers and thus has an impact in decision of management of the patients of breast cancers. BLBC is a molecular subtype of breast cancer known for its aggressive behaviour and poor prognosis and is a heterogeneous tumour, the genetic profile and molecular mechanisms are yet to be definitively characterised and are identified by expression of basal CKs. Lack of targeted therapy makes treatment of BLBC a challenging task. Further studies are needed to suggest the pathogenesis of these tumours and to develop new targeted therapy. Therefore, evaluation of subtypes of breast cancer should be incorporated in routine practice.
*Fishers exact test LVI-Lymphovascular Invasion **119 cases of IDC taken into consideration, 2 cases of Medullary carcinoma and 1 cases of Lobular carcinoma excluded; p<0.05- statistically significant.
*Fishers-exact test **Either ER or PR or Both Positive ***Both ER and PR Negative; p<0.05- statistically significant