Solid-Pseudopapillary Tumour of the pancreas (SPT) or Frantz’s tumour is a rare epithelial neoplasm and represents 2% of exocrine pancreatic tumours. Its origin remains enigmatic and they are of little known aetiopathogenesis. These tumours occur in young women and must be suspected in any case of left hypochondrium’s mass. It has a good prognosis through radical surgical excision. The five-year survival rate is around 97%. Here, the authors present a case of 26-year-old female patient, who presented to the Emergency Department with three weeks history of abdominal pain and vomiting. Physical examination did not reveal any abnormalities and her blood tests were normal. Cross-sectional imaging modalities which showed a caudal pancreatic mass with cystic and necrotico-haemorrhagic components evocative of a pseudo-papillary solid tumour of the pancreas. The patient had a distal pancreatectomy and splenectomy with good outcome.
Cystic mass, Epithelial neoplasm, Pancreas, Surgery
Case Report
A 26-year-old female patient, of North-African origin, presented to the Emergency Department with epigastralgia and left upper quadrant abdominal pain of a progressive onset associated with vomiting and a feeling of heaviness evolving for three weeks. She had no medical or surgical history and she was Gravidia0, Para0.
The physical examination did not reveal any abnormalities: She had a body temperature of 37.6°C, a blood pressure of 128/75 mm of Hg, a heart rate of 88 bpm and haemoglobin saturation of 98%. Her abdomen was non-tender. Laboratory blood tests showed a WBC count of 8960 cells/mm3, a CRP of 3 mg/L, no cytolysis, no cholestasis and no hyperbilirubinemia. The kidney function was normal (creatinine=6 mg/L, blood urea=0.2 d/L) and pancreatic test was normal (serum lipase=26 IU/L). The tumour markers (ACE, CA19-9, alpha-fetoprotein) were all within normal range.
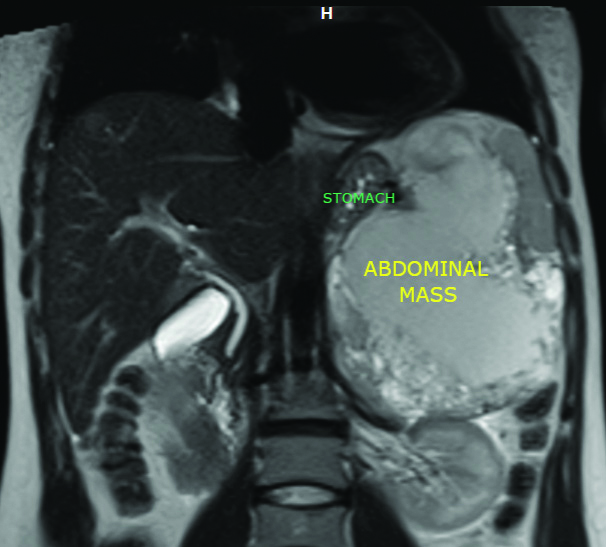
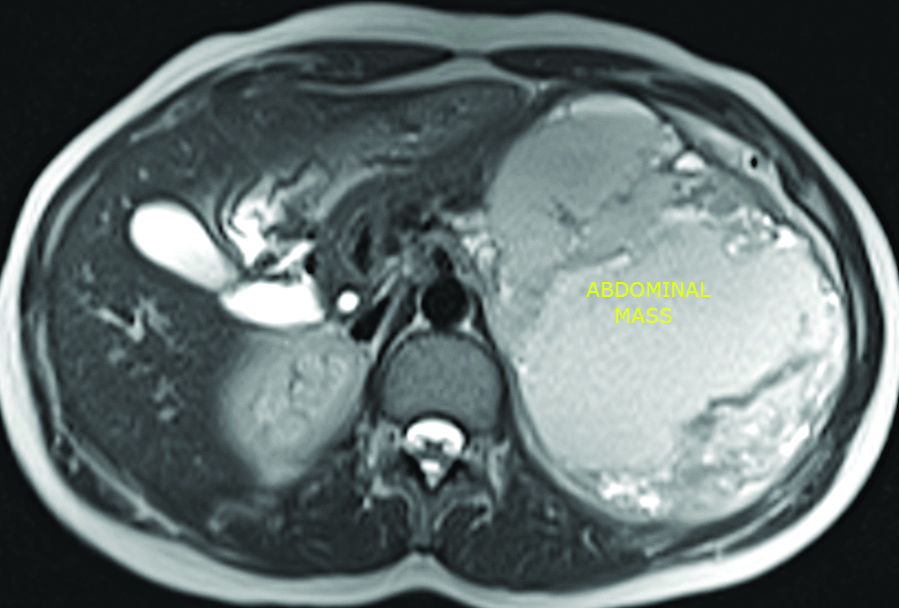
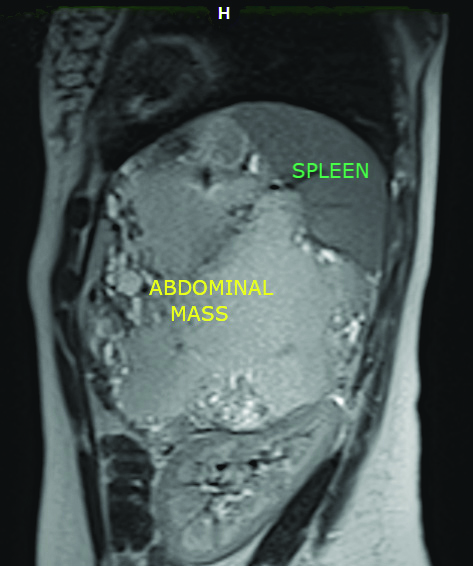
An abdominal ultrasound was performed and it showed a large heterogeneous solido-cystic mass in the rear cavity of the omentum. Contrast abdominal CT showed a tumour arising from the pancreas with 15 cm of greater diameter and cystic components. The abdominal MRI showed a caudal pancreatic mass [Table/Fig-1,2] measuring 16×15.5×11 cm with a large necrotio-haemorrhagic component and cystic formations of variable size associated with a moderately increased peripheral enhancement after gadolinium injection, occupying the hole left upper quadrant of abdomen with a mass effect on the spleen, suggestive of a pseudo-papillary solid tumour of the pancreas [Table/Fig-3].
Coronal MRI view showing the caudal pancreatic mass.
Axial MRI view showing the tumour.
Sagittal MRI view showing the tumour and the mass effect on the spleen.
Based on the cross-sectional imaging data, the decision of the multidisciplinary meeting was surgery. The exploration found a huge mass below the spleen, which pushed the stomach to the right and the left kidney down. Distal pancreatectomy and splenectomy were performed.
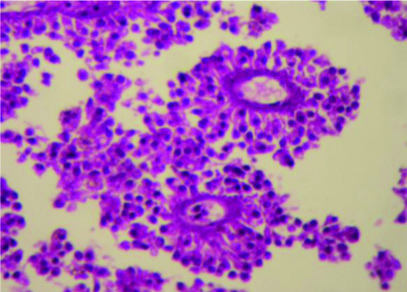
The postoperative period was uneventful. The pathology examination confirmed the diagnosis showing a poorly monomorphic tumour cells forming pseudopapillae [Table/Fig-4]. In follow-up visits, the patient had full recovery.
Tumour tissue (magnification X400) showing poorly cohesive monomorphic tumour cells radiating around blood vessels forming pseudopapillae.
Discussion
The SPT of the pancreas represents 5% of pancreas’ cystic tumours and 2% of pancreatic exocrine neoplasms [1]. This tumour is mainly seen in young women (average age 28 years) with a sex ratio of 10:1 [2].
The pathogenesis of SPT is still poorly understood. It may have hormonal origin because of the positivity of tumour’s hormonal receptors [3]. It affects more frequently the corporeal and caudal region of the pancreas [4]. This tumour is associated with a low-grade malignancy potential, however metastasis are noted in 15% of patients and recurrence rate after surgery is about 15% of cases [2,4].
Clinical presentation of this deep-seated tumour lacks specificity and the physical examination does not usually reveal typical suggestive reliable signs [5]. It may include a dull abdominal pain or signs related to the compression of the neoplasm’s surrounding digestive biliary or vascular structures in connection with the tumour [2]. A regular, firm and painless epigastric mass was found on deep palpation of the abdomen in only 30% of patients, according to the study conducted by Song H et al., [4]. It can be discovered fortuitously during a radiological examination or at the complication stage [3,5,6].
Biological examinations and tumour markers are also often non-contributory [4]. Radiological investigations play a key role in establishing the diagnosis. The abdominal ultrasound, may show a heterogeneous lesion depending on the predominant cystic or tissue portion. The abdominal CT confirms the pancreatic origin of neoplasm, objectify its heterogeneous nature as well as its local connections with vessels and other neighboring organs and provides a detailed resectability evaluation of the tumour [7]. On the other hand, vascular invasion and lymph node involvement are rare and found respectively in 6% and 9% of cases [8].
Abdominal MRI is the examination of choice, since it allows characterising the different contingents of the tumour, and indicates the presence of a capsule which usually appears as a thin hypointense rim [5]. This radiological sign associated with the high signal intensity due to the presence of haemorrhagic areas are very suggestive of an SPT [5].
Among the other evocative signs of SPT in MRI, are the large tumour size exceeding 8.6 cm on average, the corporeal caudal localisation, the areas of intratumour haemorrhages in hypersignal T1 and T2 with liquid-liquid levels, the content mixed predominantly cystic without septa and the thin capsule contained in T1 and T2 hyper signal [9]. Finally, if the radiological data are not clear and specific enough to help the diagnosis, endoscopic fine needle aspiration cytology represents another highly valuable diagnostic alternative [10].
The main differential diagnoses are other pancreatic cystic or solid tumours such as pseudocyst, microcystic adenoma and cystadenocarcinoma [5]. However, a careful analysis of the radiological semiology, mainly in MRI, in a young woman with good general condition and normal biology should be enough to evoke the diagnosis of SPT. The preoperative biopsy is intended if in doubt of diagnosis because it exposes to the risk of tumour dissemination [4,11].
The treatment is primarily based on surgical resection of the tumour. It represents the curative treatment for SPT, even in the recurrence cases, with several modalities, which essentially depend on the location, local relationships, and neoplasm size which varies between 2 and 25 cm [3]. These modalities vary from simple enucleation to total pancreatectomy [7]. In the event of neighboring organs, invasion or metastatic lesions (among other nodules of peritoneal carcinosis) the surgical technique must be completed by an extended resection and metastasectomy. Lymph node dissection is unnecessary in this type of tumour and the indication for chemotherapy or radiotherapy remains questionable [3].
The pathology examination confirms the diagnosis. Macroscopically, the tumour mass is generally delimited by a fibrous capsule with a solid-cystic and haemorrhagic appearance inside. Histologically, the tumour is divided into two components: solid peripheral and central pseudo-papillary. Atypia such as giant cells around cholesterol crystals or clumps of foaming histiocytes are exceptional. Malignancy criteria include perineural invasion, adjacent structures invasion, lymph node or distant metastases and vascular emboli [12]. Immunohistochemical study usually reveals positivity for anti CD10, vimentin, neuron specific enolase, alpha 1-antitrypsin, E cadeine, Betacaternine and anti-progesterone antibodies [4,7]. In this case, the SPT was classified as pseudo-papillary and solid carcinoma.
Survival at five years is around 97% with a significant recurrence rate of 15% [3] highlighting the interest of a regular postoperative follow-up with radiological monitoring.
Conclusion(s)
Frantz’s tumour is rare. The diagnosis is helped by the contribution of clinical, biological and radiological data and confirmed by the anatomo-pathological study. Its prognosis is better than that of other pancreatic tumours, which justifies radical and curative surgery.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jun 01, 2020
Manual Googling: Jul 21, 2020
iThenticate Software: Sep 28, 2020 (5%)
[1]. Canzonieri V, Berretta M, Buonadonna A, Vasquez E, Barbagallo E, Bearz A, Solid pseudopapillary tumour of the pancreasThe Lancet Oncology 2003 4(4):255-56.10.1016/S1470-2045(03)01038-6 [Google Scholar] [CrossRef]
[2]. Peng-FeiYu Y, Zhen-Hua H, Xin-Bao W, Jian-Min G, Xiang-Dong C, Yun-Li Z, Solid pseudopapillary tumour of the pancreas: A review of 553 cases in Chinese literatureWorld J Gastroenterol 2010 16(10):1209-14.10.3748/wjg.v16.i10.120920222163 [Google Scholar] [CrossRef] [PubMed]
[3]. Cheng-Hong P, Dong-Feng C, Guang-Wen Z, Wei-Ping Y, Zong-Yuan T, Ruo-Qing L, The solid-pseudopapillary tumour of pancreas: The clinical characteristics and surgical treatmentJournal of Surgical Research 2006 131(2):276-82.10.1016/j.jss.2005.11.58516457845 [Google Scholar] [CrossRef] [PubMed]
[4]. Song H, Dong M, Zhou J, Sheng W, Zhong B, Gao W, Solid pseudopapillary neoplasm of the pancreas: clinicopathologic feature, risk factors of malignancy, and survival analysis of 53 cases from a single centerBiomed Res Int [Internet] 2017 2017:5465261Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5637868/10.1155/2017/546526129094047 [Google Scholar] [CrossRef] [PubMed]
[5]. Francis W, Goldenberg E, Adsay N, Steffes C, Webber J, Solid-pseudopapillary Tumours of the pancreas: Case report and literature review [Internet]Current Surgery 2006 63(6):469-72.[cited 2020 May 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/17084780/10.1016/j.cursur.2006.06.01017084780 [Google Scholar] [CrossRef] [PubMed]
[6]. Klimstra D, Wenig B, Heffess C, Solid-pseudopapillary tumour of the pancreas: A typically cystic carcinoma of low malignant potential [Internet]Seminars in Diagnostic Pathology 2000 17(1):66-80.[cited 2020 May 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/10721808/ [Google Scholar]
[7]. Papavramidis T, Papavramidis S, Solid pseudopapillary tumours of the pancreas: Review of 718 patients reported in english literature [Internet]Journal of the American College of Surgeons 2005 200(6):965-72.[cited 2020 May 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/15922212/10.1016/j.jamcollsurg.2005.02.01115922212 [Google Scholar] [CrossRef] [PubMed]
[8]. Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, A Systematic Review of Solid-Pseudopapillary Neoplasms: Are these rare lesions?Pancreas 2014 43(3):331-37.10.1097/MPA.000000000000006124622060 [Google Scholar] [CrossRef] [PubMed]
[9]. Yu MH, Lee JY, Kim MA, Kim SH, Lee JM, Han JK, MR Imaging features of small solid pseudopapillary tumours: Retrospective differentiation from other small solid pancreatic tumours [Internet]AJR. American Journal of Roentgenology 2010 195(6)[cited 2020 May 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/21098190/10.2214/AJR.10.445221098190 [Google Scholar] [CrossRef] [PubMed]
[10]. Botello-Hernández Z, Fuentes-Reyes RA, Hernández-González M, Mosqueira-Mondragón C, Pérez-Torres E, Chapa-Azuela O, Frantz’s tumour. Unusual presentation in two adolescentsRevista Médica del Hospital General de México 2018 81(3):122-26.10.1016/j.hgmx.2017.06.003 [Google Scholar] [CrossRef]
[11]. Fais P, Carricaburu E, Sarnacki S, Berrebi D, Orbach D, Baudoin V, Is laparoscopic management suitable for solid pseudo-papillary tumours of the pancreas? [Internet]Pediatric Surgery International 2009 25(7):617-21.[cited 2020 May 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/19479267/10.1007/s00383-009-2388-919479267 [Google Scholar] [CrossRef] [PubMed]
[12]. Mirminachi B, Farrokhzad S, Sharifi AH, Nikfam S, Nikmanesh A, Malekzadeh R, Solid pseudopapillary neoplasm of pancreas; a case series and review literatureMiddle East J Dig Dis 2016 8(2):102-08.10.15171/mejdd.2016.1427252816 [Google Scholar] [CrossRef] [PubMed]